In either acid or base, 3-cyclohexenone comes to equilibrium with 2-cyclohexenone: (a) Explain why the equilibrium favors
Question:
In either acid or base, 3-cyclohexenone comes to equilibrium with 2-cyclohexenone: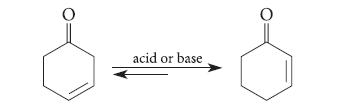
(a) Explain why the equilibrium favors the α,β-unsaturated ketone over its β,γ-unsaturated isomer.
(b) Give a mechanism for this reaction in aqueous NaOH.
(c) Give a mechanism for the same reaction in dilute aqueous H2SO4.
(d) Is the equilibrium constant for the analogous reaction of 4-methyl-3- cyclohexenone expected to be greater or smaller? Explain.
Transcribed Image Text:
acid or base O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a The equilibrium favors the aunsaturated isomer because it is conjugated Conjugation is a stabilizi...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The incorrect regarding the following structure is NH2 OH HO-P N. OH Select one O a Analogue of deoxycytidine 5-monophosphate Ob Denved from isoster replacememnt with deoxycytidine 5-monophosphate Oc...
-
The grocery retail industry across America has always tended to involve healthy competition among the various firms in it. (a) Identify the various business competitors in this industry across any...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Firms raise capital from investors by issuing shares in the primary markets. Does this imply that corporate financial managers can ignore trading of previously issued shares in the secondary market?
-
What is the basic accounting equation? Discuss.
-
What constitutes contemporary GAAP?
-
How might a hacker access and manipulate a digital device for illegal purposes? Are the Internet of Things (IoT) devices at risk for hacker access and manipulation?
-
All That Blooms provides environmentally friendly lawn services for homeowners. Its operating costs are as follows. Depreciation ......... $1,400 per month Advertising ........ $200 per month...
-
You are given the following algorithm written in pseudocode where the entries of the input array A and the input k are positive integer numbers. Also, assume that array A has sufficiently large...
-
(a) The resonance structures shown in part (a) of Fig. P22.76 can be written for an ,-unsaturated carboxylic acid. Would this type of resonance interaction increase or diminish the acidity of an ,-...
-
In 3-methyl-2-cyclohexenone the eight hydrogens H a , H b , H c , and H d can be exchanged for deuterium in CH 3 O/CH 3 OD. (a) Write curved-arrow mechanisms for the basecatalyzed exchange of...
-
Reconsider Prob. 770. Using EES (or other) software, investigate the effect of the wind velocity on the surface temperature of the wire. Let the wind velocity vary from 10 km/h to 80 km/h. Plot the...
-
What are three unique benefits of the Palo Alto Networks Content-ID? Detecting and preventing known and unknown threats in a single pass. Proactively identifying and defending against unknown, new,...
-
One person has carried out the roles of CEO and chairman since the incorporation of Value Pty (Ltd) 10 years ago. Recently, analysts have begun to criticise this policy explaining that since the CEO...
-
Developing a diverse workforce has always been a challenging objective. Considering gender, race, ethnicity, religion and sexual preference are but some of the areas of concern to consider. We have...
-
Compare and contrast the traditional UCR and the enhanced UCR/NIBRS program, discussing three of the differences between the two programs. Discuss the limitations of the UCR system that led to the...
-
Please read and answer: The Centennial Company manufactured small electrical appliances for the consumer market. Its major product line consisted of several models of electric shavers for both men...
-
Long Beach Pharmaceutical Company has two divisions, which reported the following results for the most recent year. Required: Which was the more successful division during the year? Think carefully...
-
Discuss the information available from the following techniques in the analysis of inorganic pigments used in antique oil paintings: (i) Powder X-ray diffraction, (ii) Infrared and Raman...
-
Write a mechanism that explains the following reaction. OH 2, NaHCO3
-
Write a mechanism for the following reaction. OH H SO. H2O
-
Write a mechanism that explains formation of the products shown in the following reaction. Br, NaCI, H20o Rr CL
-
You have just received a bonus of 15,000 euros and have decided that you want to invest the money. After discussing your options with your bank, you have narrowed it down to two options: A. A savings...
-
Where on the HHI index would an industry with five companies with the following market shares be categorized? Firm A 24% Firm B 23% Firm C 20% Firm D 15% Firm E 18%
-
Question: How does the saving rate of middle-career individuals compare with that of retirees? Please explain the economic reasoning.

Study smarter with the SolutionInn App


