Make a model of chair cyclohexane corresponding to the leftmost model in Fig. 7.4. Raise carbon-4 so
Question:
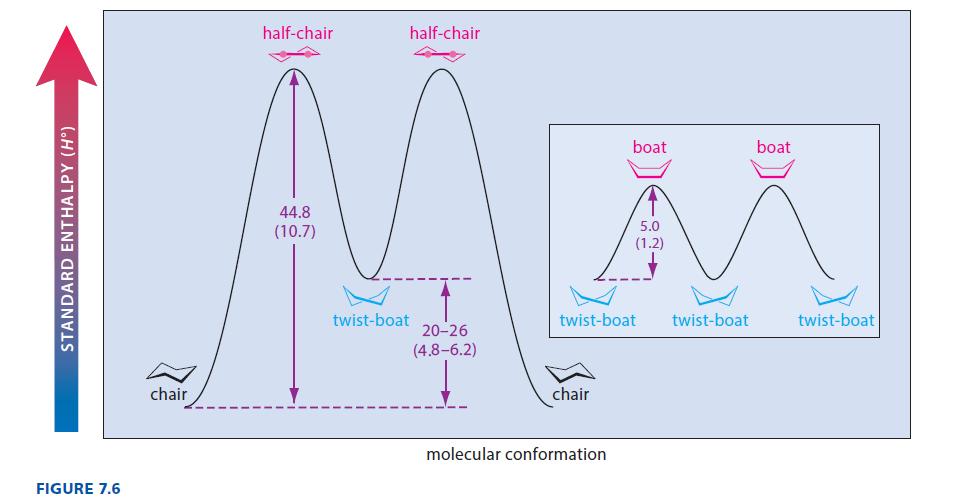
Make a model of chair cyclohexane corresponding to the leftmost model in Fig. 7.4. Raise carbon-4 so that carbons 2–5 lie in a common plane. This is the half-chair conformation of cyclohexane, and it is the transition state for the interconversion of the chair and twist-boat conformations. (Notice the position of this conformation on the energy diagram of Fig. 7.6.) Give two reasons why the half-chair conformation is less stable than the chair or twist-boat conformation.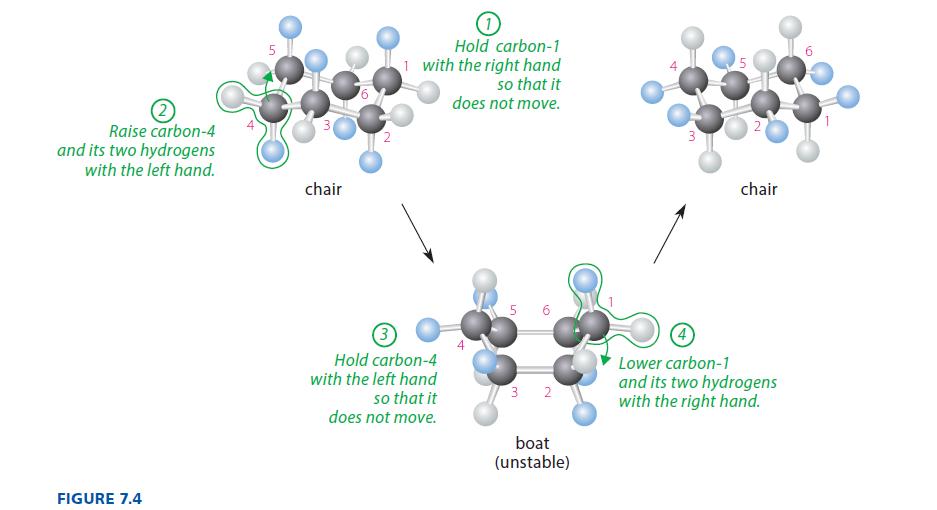
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





