Methanol containing the oxygen isotope 18 O is allowed to react, in separate reactions, with each of
Question:
Methanol containing the oxygen isotope 18O is allowed to react, in separate reactions, with each of the acid chlorides shown in Fig. P25.29a–c, and each of the resulting compounds A, C, and E is treated with one equivalent of sodium hydroxide. This treatment regenerates methanol in all three cases, along with hydrolysis products B, D, and F, respectively. Identify the compounds A–F and account for the distribution of the isotope in all three cases. Specifically, why is isotopically substituted methanol formed in reaction (b) but not in reactions (a) and (c)?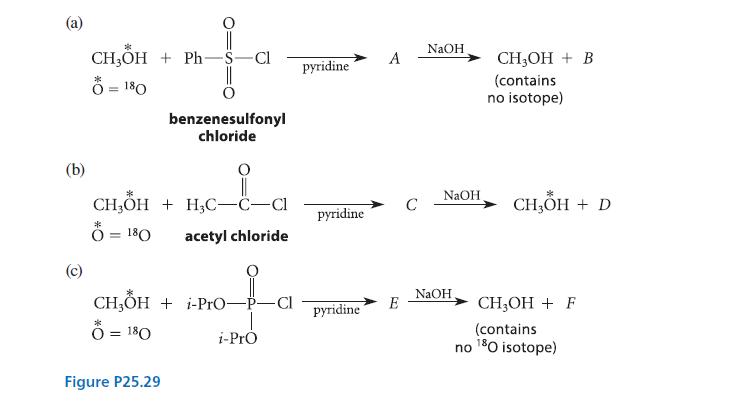
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





