The first demonstration of the stereochemistry of the S N 2 reaction was carried out in 1935
Question:
The first demonstration of the stereochemistry of the SN2 reaction was carried out in 1935 by Prof. E. D. Hughes and his colleagues at the University of London. They allowed (R)-2-iodooctane to react with radioactive iodide ion (*I¯).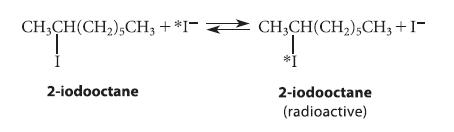
The rate of substitution (rate constant kS) was determined by measuring the rate of incorporation of radioactivity into the alkyl halide. The rate of loss of optical activity from the alkyl halide (rate constant k°) was also determined under the same conditions.
(a) What ratio k°/kS is predicted for each of the following stereochemical scenarios:
(1) Retention;
(2) Inversion;
(3) Equal amounts of both retention and inversion? Explain.
(b) The experimental rate constants were found to be as follows:
![]()
Which scenario in part (a) is consistent with the data?
Step by Step Answer:






