The aconitase-catalyzed addition of water to cis-aconitate in the citric acid cycle occurs with the following stereochemistry.
Question:
The aconitase-catalyzed addition of water to cis-aconitate in the citric acid cycle occurs with the following stereochemistry. Does the addition of the OH group occur on the Re or the Si face of the substrate? What about the addition of the H? Does the reaction have syn or antistereochemistry?
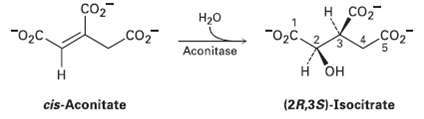
Transcribed Image Text:
н со Но "02c. "O2C. co2 3. Aconitase H OH он (2R,3S)-Isocitrate cis-Aconitate 3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
Addition of OH takes place on the Re face of aconi...View the full answer

Answered By

Ishrat Khan
Previously, I have worked as an accounting scholar at acemyhomework, and have been tutoring busines students in various subjects, mostly accounting. More specifically I'm very knowledgeable in accounting subjects for college and university level. I have done master in commerce specialising in accounting and finance as well as other business subjects.
5.00+
134+ Reviews
427+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The first step in the citric acid cycle is reaction of oxaloacetate with acetyl CoA to give citrate. Propose a mechanism, using acid or base catalysis asneeded. "O2C. 02C H .co2 CO SCOA Citrate...
-
Which of the substances in the citric acid cycle are tricarhoxylic acids, thus giving the cycle its alternative name?
-
Isocitric acid, an intermediate in the citric acid cycle of food metabolism, has the systematic name (2R, 3S)-3-carboxy-2-hydroxypentanedioic acid. Draw the Structure.
-
Foreman Publishing Companys income for the most recent quarter was $500,000, and the average net book value of assets during the quarter was $1.5 million. If the company has a required rate of return...
-
Consider a bar 1 m long that expands 0.6 cm when heated. Show that when similarly heated, a 100-m bar of the same material becomes 100.6 m long.
-
Determine the moments at A, B, and C by the moment-distribution method. Assume the supports at A and C are fixed and a roller support at B is on a rigid base. The girder has a thickness of 4 ft. Use...
-
What are the main causes of expatriate failure?
-
Jackson Company, which uses the high-low method to analyze cost behaviour, has determined that machine hours best predict the companys total utilities cost. The companys cost and machine hour usage...
-
Before they become eligible to receive CPP retirement benefits, Lian and Cheng have calculated that they will have an essential expense gap of $1,450 per month between age 55 and age 60. Assuming a...
-
A conflict has broken out between India and Pakistan over rising tensions in the Kashmir region. Because of the instability, many investors are moving their money into more stable currencies. This...
-
Lactic acid buildup in tired muscles results from reduction of pyruvate. If the reaction occurs from the Re face, what is the stereochemistry of theproduct? OH CH3CHCO2 H3C CO2 Lactate Pyruvate
-
Which of the following structures are identical? (Yellow-green =Cl) (a) (b) (c) (d)
-
Given find each product, if possible. CB 5 -2 B = | 0 -2 4 -5 4 -2, and C = 0 3 6 3 ||
-
(10 points) Marketing Message: Describe the message you plan to communicate to the target market. This should be a focused message that explains the benefits of the product/event in a way that...
-
A pendulum consists of a bob with mass 5 0 g and a string with length 3 0 cm . It is released from rest from an angle of 1 0 to the vertical. What is the maximum speed of the bob?
-
(10 points) 3. Discuss the impact the Internet has on marketing and businesses. Include three ways marketing has changed with the advent of the Internet and three ways businesses have adapted with...
-
What role do middleware components such as device drivers, communication stacks, and protocol implementations play in the development of embedded operating systems, and how do they facilitate...
-
You found the following information about Markus Inc. (in millions $) for the year 2022: NOPAT = $28; after-tax interest = $4; beginning equity = 180, ending equity = 190. Using average equity...
-
Recreational facilities run by a governmental unit and financed on a user-charge basis would be accounted for in which fund? a. General b. Trust c. Enterprise d. Capital projects
-
Create a data model for one of the processes in the end-of-chapter Exercises for Chapter 4. Explain how you would balance the data model and process model.
-
A 1.25-g gas sample occupies 663 mL at 25 C and 1.00 atm. What is the molar mass of the gas? a) 0.258 g/mol b) 0.0461 g/mol c) 3.87 g/mol d) 46.1 g/mol
-
Explain the following findings. (a) One full equivalent of base must be used in the Claisen or Dieckmann condensation. (b) Ethyl acetate readily undergoes a Claisen condensation in the presence of...
-
A student, Cringe Labrack, has suggested each of the faulty synthetic procedures shown in Fig. P22.73. Explain why each one cannot be expected to work. NadEt CHAI NaOEt PhBt a) CH,CH CO,Et (c OH CH...
-
A student, Cringe Labrack, has suggested each of the faulty synthetic procedures shown in Fig. P22.73. Explain why each one cannot be expected to work. NadEt CHAI NaOEt PhBt a) CH,CH CO,Et (c OH CH...
-
1 B C Use Excel to journalize the adjusting entries. Use the blue shaded areas for inputs. (Always use cell references and formulas where appropriate to receive full credit. If you copy/paste from...
-
A. Make each pair of radicals similar by reducing the radicand. 1. 2,8 2. 3.24 3. 45,125 4. 3,96 5. 27.75 6. 48,12 7. 28,63 8. 4/32,1/162 9. 40,135 10. 8.200 B. Referring to each pair in letter A, do...
-
Assume that you are the president of Highlight Construction Company. At the end of the first year of operations (December 31), the following financial data for the company are available: Cash...

Study smarter with the SolutionInn App


