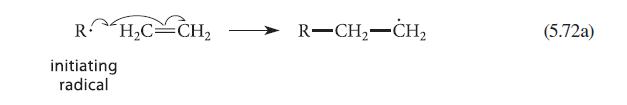
The mechanism for the free-radical polymerization of ethylene shown in Eqs. 5.72ac (pp. 217218) is somewhat simplified
Question:
The mechanism for the free-radical polymerization of ethylene shown in Eqs. 5.72a–c (pp. 217–218) is somewhat simplified because it does not account for the observation that low-density polyethylene (LDPE) contains a significant number of branched chains. (The branching accounts for the low density of LDPE.) It is believed that the first step that leads to branching is an internal hydrogen abstraction reaction that occurs within the growing polyethylene chain, shown in Fig. P5.45.
(a) Use bond dissociation energies to show that this process is energetically favorable.
(b) Show how this reaction can lead to a branched polyethylene chain.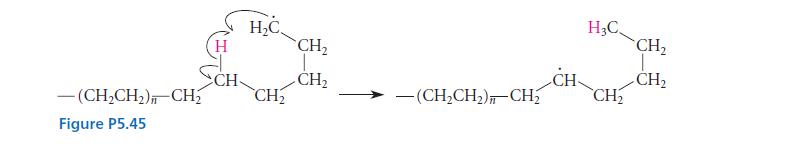


Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





