Two general mechanisms (or various versions of them) for alkene metathesis were originally considered. In the first
Question:
Two general mechanisms (or various versions of them) for alkene metathesis were originally considered. In the first (pairwise) mechanism, shown in Fig. P18.88, the catalyst brings about cyclobutane formation between two alkenes. The second mechanism involves metallacycle formation.
(a) The metathesis reaction of cyclopentene and (E)-2-pentene gives three products (not counting stereoisomers): 2,7-nonadiene, 2,7-decadiene, and 3,8-undecadiene, in a ratio of 1 : 2 : 1. Show how this result can be used to decide between the two mechanisms. Ignore self-metathesis products of cydopentene and (E)-2 pentene.
(b) What result is predicted by the two mechanisms for the ratio of the three ethylene products in the reaction shown in Fig. P18.88b? Assume that once an ethylene molecule is formed, it undergoes no further reaction.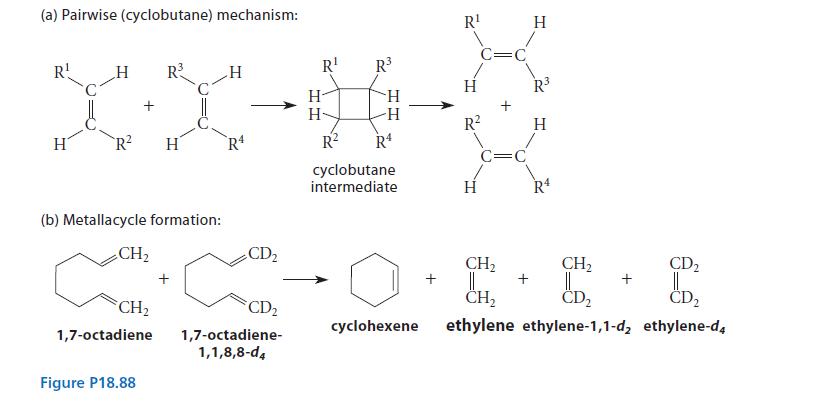
Step by Step Answer:






