Use the principles of Sec. 1.3B to predict the geometry of BF 3 . What hybridization of
Question:
Use the principles of Sec. 1.3B to predict the geometry of BF3. What hybridization of boron is suggested by this geometry? Draw an orbital diagram for hybridized boron similar to that for the carbons in ethylene shown in Fig. 4.3, and provide a hybrid orbital description of the bonding in BF3.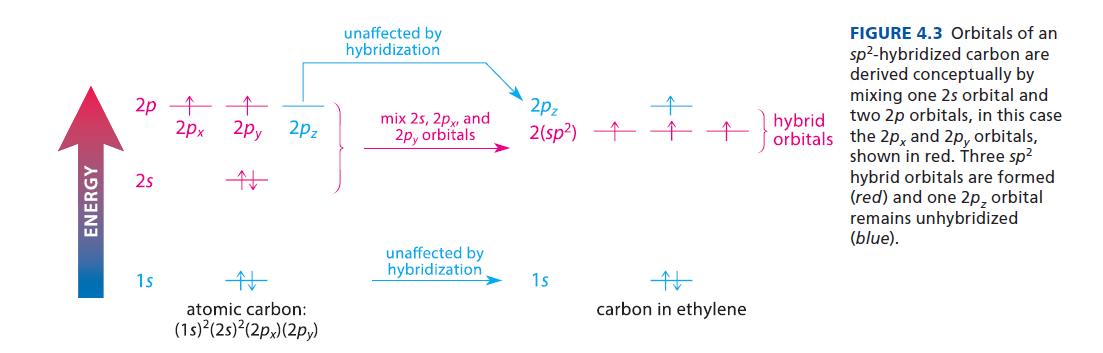
Transcribed Image Text:
ENERGY 2p ↑ 2s ↑ 2px 2py ↑ 1s 2Pz atomic carbon: (1s)²(2s)²(2px) (2py) unaffected by hybridization mix 2s, 2px, and 2p, orbitals unaffected by hybridization 2pz 2(sp²) ↑ 1s # carbon in ethylene FIGURE 4.3 Orbitals of an sp²-hybridized carbon are derived conceptually by mixing one 2s orbital and hybrid two 2p orbitals, in this case orbitals the 2px and 2p, orbitals, shown in red. Three sp² hybrid orbitals are formed (red) and one 2p, orbital remains unhybridized (blue).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Boron trifluoride trifluoroborane BF 3 is trigonal planar with FBF bond angles of 120 Because hybrid...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the polygon-and-circle method to draw an orbital diagram for each of the following compounds. (a) (b)
-
The central carbon atom of an allene is a member of two double bonds, and it has an interesting orbital arrangement that holds the two ends of the molecule at right angles to each other. (a) Draw an...
-
5. (12 points total) Let A = Rmxn be a full rank matrix with m n. Such matrices were the object of focus at the end of Unit 3, when we solved least-squares problems. In that unit, we introduced two...
-
Calculate the half life potential for Zn electrode is 0.01M Zn(NO 3 ) 2. Given that Zn 2+ + 2e= Zn E o = 0.763
-
Presented here are several transactions and events of the General Fund of Johnson County. All transactions and events relate to calendar year 2012. 1. Estimated revenues from the following sources...
-
Suppose a U.S. parent owes $5 million to its English affiliate. The timing of this payment can be changed by up to 90 days in either direction. Assume the following effective annualized after-tax...
-
What are some indicators that a digital device has been infected?
-
The accountant for Teresa's Dress Shop prepared the following cash budget. Teresa's desires to maintain a cash cushion of $14,000 at the end of each month. Funds are assumed to be borrowed and repaid...
-
Amazon, Inc. Presentation Your chief executive officer (CEO) has asked you to present the company's (Amazon, Inc.) process on making decisions under risks and uncertainty at the annual shareholders'...
-
The enantiomeric resolution in Fig. 6.16 used the chiral stationary phase (CSP) in Eq. 6.11. How would the enantiomeric resolution in Fig. 6.16 be affected if (a) The enantiomer of the CSP in Eq....
-
Malonic acid has two carboxylic acid groups and consequently undergoes two ionization reactions. The pK a for the first ionization of malonic acid is 2.86; the pK a for the second is 5.70. The pK a...
-
The FASB ASC contains guidance on how to account for equity investments when accumulated losses by the investee have resulted in the investment account of the investor being reduced to zero. Find,...
-
Heather has just bought a bond that will mature in 5 years for 300, with a 320 par value and a coupon rate of 10% paid semiannually. What should the value of this bond be if the required return on...
-
A 2-year Treasury bond currently offers a 6% rate of return. A 3-year Treasury bond offers a 7% rate of return. Under the expectations theory, what rate of return do investors expect a 2-year...
-
Suppose you purchase a 30-year Treasury bond with a 6% annual coupon, initially trading at par. In 10 years time, the bonds yield to maturity has risen to 7% (EAR). a. If you sell the bond now, what...
-
Transaction costs Assume that you use a TD Ameritrade brokerage account and place your stock trades through the Interactive Voice Response (IVR) Phone System, which charges a $34.99 commission per...
-
Jonathan Harper currently has 1,000 that he can spend today on novelty yarn costing 20 a bundle. Alternatively, he could invest the 1,000 in a Bank of England bond that pays 6% nominal rate of...
-
List cash flows that a hospital is likely to incur in conjunction with the installation of a new CAT scanner.
-
Draw the major product for each of the following reactions: (a) (b) (c) 1) 9-BBN 2) H2O2, NaOH 1) Disiamylborane 2) H20, NaOH
-
What condensation product would you expect to obtain by treatment of the following substances with sodium ethoxide in ethanol? (a) Ethyl Butanoate (b) Cyclopentanone (c) 3, 7-Nonanedione (d)...
-
In the mixed Claisen reaction of cyclopentanone with ethyl format, a much higher yield of the desired product is obtained by first mixing the two carbonyl components and then adding base, rather than...
-
Give the structures of the possible Claisen condensation products from the following reactions. Tell which, if any, you would expect to predominate in each case. (a) CH3CO2Et + CH3CH2CO2Et (b)...
-
Evaluate the integral (use c for the constant of integration I 1) 2) x-2x-1 (x-1)2(x+1) dx 7x3+3x+35x +3 (x+1)(x+5) 13 3) Sx-125 dx dx ) ex (ex-7) (ex+1) dx
-
4. Either give an example, or state why so such example exists. a) An unbounded sequence that has a convergent subsequence b) A Cauchy sequence that has a divergent subsequence c) A Cauchy sequence...
-
A tank full of water is designed with ends in the shape of an isosceles triangle with height 8 meters and width, at the top, of 4 meters. Find the hydrostatic force on one end of the tank.

Study smarter with the SolutionInn App


