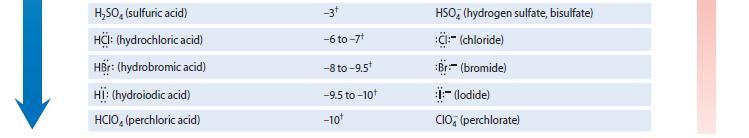
Write an equation for each of the following equilibria, and use Table 3.1 to identify the pK
Question:
Write an equation for each of the following equilibria, and use Table 3.1 to identify the pKa value associated with the acidic species in each equilibrium.
(a) Ammonia acting as a base toward the acid water
(b) Ammonia acting as an acid toward the base water Which of these reactions has the larger Keq and therefore is more important in an aqueous solution of ammonia?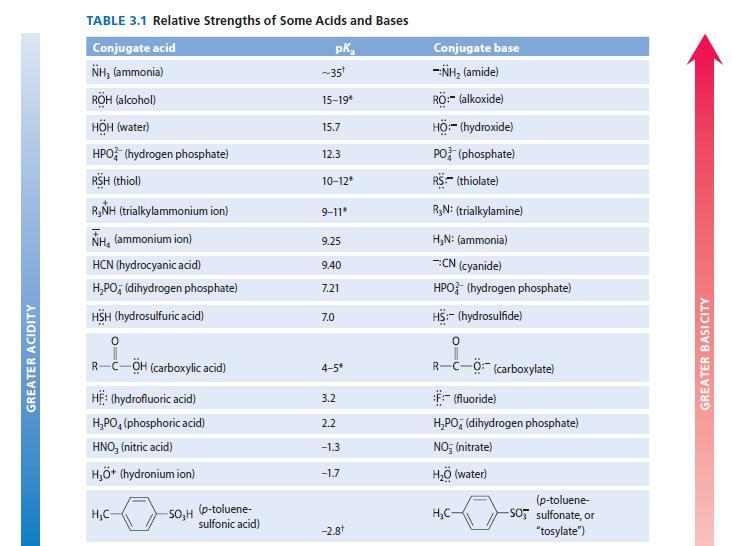
Transcribed Image Text:
GREATER ACIDITY TABLE 3.1 Relative Strengths of Some Acids and Bases Conjugate acid NH, (ammonia) RÖH (alcohol) HÖH (water) HPO (hydrogen phosphate) RSH (thiol) R,NH (trialkylammonium ion) NH, (ammonium ion) HCN (hydrocyanic acid) H₂PO, (dihydrogen phosphate) HSH (hydrosulfuric acid) R-C-OH (carboxylic acid) HE: (hydrofluoric acid) H₂PO (phosphoric acid) HNO, (nitric acid) H₂O* (hydronium ion) H₂C- -SO₂H (p-toluene- sulfonic acid) pK₂ ~35t 15-19 15.7 12.3 10-12* 9-11* 9.25 9.40 7.21 7.0 4-5* 3.2 2.2 -1.3 -1.7 -2.8+ Conjugate base -NH₂ (amide) RÖ:- (alkoxide) HÖ:- (hydroxide) PO (phosphate) RS (thiolate) R₂N: (trialkylamine) H₂N: (ammonia) -:CN (cyanide) HPO (hydrogen phosphate) HS:- (hydrosulfide) R-C-Ö: (carboxylate) F:-(fluoride) H₂PO (dihydrogen phosphate) NO; (nitrate) H₂O (water) H₂C- (p-toluene- -SO sulfonate, or "tosylate") GREATER BASI CITY
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Ammonia acting as a ba...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write an equation for each of the following reactions: a. 2-methyl-2-butanol + HCl b. 3-pentanol + Na c. Cyclohexanol + PBr3 d. 2-phenylethanol + SOCl2 e. 1-methylcyclopentanol + H2SO4, heat f....
-
Write an equation for each of the following reactions. If no reaction occurs, say so. a. methyl propyl ether + excess HBr (hot) - b. dibutyl ether + boiling aqueous NaOH - c. ethyl ether + cold...
-
Write an equation for each of the following reactions: a. 2-butene + HCl b. 3-hexene + HI c. 4-methylcyclopentene + HBr
-
A nutritionally defective E. coli strain grows only on a medium containing thymine, whereas another nutritionally defective strain grows only on a medium containing leucine. When these two strains...
-
Why might critics think it is dangerous to permit the FAA to perform both functions?
-
Robinson Company has two products, A and B. Robinsons budget for August follows: Master budget Product A Product B Total Sales $240,000 $300,000 $540,000 Variable costs 140,000 180,000 320,000...
-
What are the different types of consulting and litigation support activities for fraud and forensic accounting professionals?
-
The Molokai Nut Company (MNC) makes four different products from macadamia nuts grown in the Hawaiian Islands: chocolate-coated whole nuts (Whole), chocolate-coated nut clusters (Cluster),...
-
4. Consider a quantum system Q described by a Hilbert space H. (a) Suppose we are given a subspace Ho of H and a linear map from kets in Ho to others in h. That is, Vo)), in a linear way. This map...
-
The basicities of conjugate bases A increase with increasing pK a of the conjugate acids AH. How do the basicities of conjugate bases A change with increasing pK b ?
-
Using the pK a values in Table 3.1, calculate the equilibrium constant for each of the following reactions. (a) NH 3 acting as a base toward the acid HCN (b) F acting as a base toward the acid HCN...
-
Identify the set as finite or infinite. Then determine whether 10 is an element of the set. {x|x is a natural number greater than 11}
-
The accounting clerk for Smith Company mistakenly posted a closing entry to an expense account twice. How will this error affect the post-closing trial balance?
-
Many employers are recognizing the bottom-line benefits of allowing employees in certain positions to work from home: telecommuting. The firm benefits from decreased operational costs. But regarding...
-
hypothetically imagine data analytics and AI is completely wiped out from the world for 24 hours. envision this world and explain what things do you see gwtting affected . what would be differnent in...
-
Identity problems that are associated with vitamin C deficiency and then identify foods that are good sources of vitamin C?
-
What should financial records provide to the clinic? In your opinion.
-
Contrast how a market system and a command economy try to cope with economic scarcity.
-
Information graphics, also called infographics, are wildly popular, especially in online environments. Why do you think infographics continue to receive so much attention? How could infographics be...
-
Draw an energy diagram for a two-step exergonic reaction whose second step is faster than its first step.
-
Draw an energy diagram for a reaction with Keq = 1. What is the value of G in this reaction?
-
The addition of water to ethylene to yield ethanol has the following thermodynamic parameters: (a) Is the reaction exothermic or endothermic? (b) Is the reaction favorable (spontaneous) or...
-
What are the theoretical differences between sql and nosql? What are the technical differences between sql and nosql?
-
Use the resources here, or find some on your own, and let's discussion what NoSQL is, why it is used, and how can we manage databases with NoSQL - if we can? If you had to choose, would you use SQL...
-
Consider your industry -- how does The Internet of Things (IoT) and Big Data currently play into your job, your organization and the industry? Are these two things connected and if so, how? If not,...

Study smarter with the SolutionInn App


