Question: As we will learn in Chapter 21, treating a lactone (a cyclic ester) with sodium hydroxide will initially produce an anion: This anion rapidly undergoes
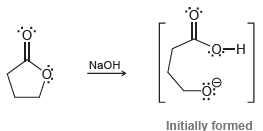
This anion rapidly undergoes an intramolecular proton transfer, in which the negatively charged oxygen atom abstracts the nearby acidic proton. Draw the product of this intramolecular acid-base process, and then identify which side of the equilibrium is favored. Explain your answer.
Q=H NaOH Initially formed
Step by Step Solution
3.57 Rating (161 Votes )
There are 3 Steps involved in it
The equilibrium fav... View full answer

Get step-by-step solutions from verified subject matter experts


