Azulene exhibits an appreciable dipole moment, and an electrostatic potential map indicates that the five-membered ring is
Question:
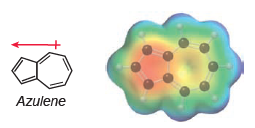
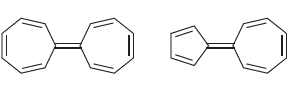
a) In Chapter 2, we saw that a resonance structure will be insignificant if it has carbon atoms with opposite charges (C- and C+). Azulene represents an exception to this rule, because some resonance structures (with C- and C+) exhibit aromatic stabilization. With this in mind, draw structures of azulene and use them to explain the observed dipole moment.
b) Based on your explanation, determine which compound is expected to exhibit a larger dipole moment.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





