Question: Consider the three compounds shown below and then answer the questions that follow: a) Which two compounds are constitutional isomers? b) Which compound contains a
a) Which two compounds are constitutional isomers?
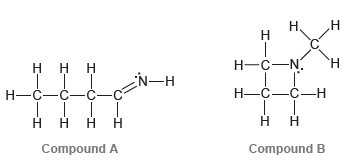
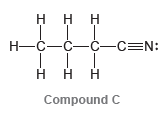
b) Which compound contains a nitrogen atom with trigonal pyramidal geometry?
c) Identify the compound with the greatest number of σ bonds.
d) Identify the compound with the fewest number of σ bonds.
e) Which compound contains more than one π bond?
f) Which compound contains an sp2-hybridized carbon atom?
g) Which compound contains only sp3-hybridized atoms (in addition to hydrogen atoms)?
h) Which compound do you predict will have the highest boiling point? Explain.
H. -N. `H Compound A Compound B DN: -C-C-C=N: Compound C
Step by Step Solution
3.45 Rating (168 Votes )
There are 3 Steps involved in it
a Compound A and Compound B b ... View full answer

Get step-by-step solutions from verified subject matter experts


