For each of the following processes predict the sign of ÎS for the reaction. In other words,
Question:
a.
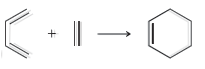
b.
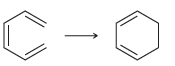
c.
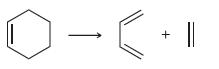
d.
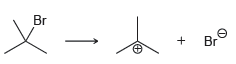
e.
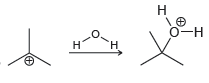
f.
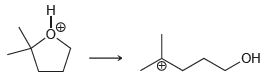
Transcribed Image Text:
||
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
a S sys is expected to be negative a decrease in entropy because tw...View the full answer

Answered By

ADHITHYA NARAYANAN
I have cleared competitive exams like GATE and JEST. I also have online problem solving experiences which would come good here as well. I have experience in tutoring in Chegg and BNED platforms.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the sign of ÎS accompanying this reaction. Explain your choice.
-
The process descriptions, process flow diagrams, and technical data for processes used commercially in 38 chemical industries. For each of the following processes, draw a block-flow diagram of just...
-
For each of the following processes, indicate whether the signs of S and H are expected to be positive, negative, or about zero. (a) A solid sublimes. (b) The temperature of a sample of Co(s) is...
-
Diagonalize the matrices, if possible. The eigenvalues are as follows: (11) = 1, 2, 3; (12) = 1, 4; (13) = 5, 1; (14) = 3, 4; (15) = 3, 1; (16) = 2, 1. 3 4 6 1
-
Q1) what is the role of the project manager in the selection of the project? What criteria does the project manager use to select the project, and how are these criteria derived? Q2) List and briefly...
-
A small business hires a consultant to predict the value of weekly sales of their product if their weekly advertising is increased to \(\$ 2000\) per week. The consultant takes a record of how much...
-
Consider the regression models described in Example 8.4. Example 8.4 a. Graph the response function associated with Eq. (8.10). Equation (8.10) b. Graph the response function associated with Eq....
-
This problem continues the Draper Consulting, Inc., situation from Problem 12-45 of Chapter 12. In October, Draper has the following transactions related to its common shares: Oct 1 Draper...
-
How does the standard define relevance and reliability? What must an auditor do if conditions indicate that a document may not be authentic or the terms in a document have been modified but that the...
-
Ivy Company purchased land and a building on January 1, 2017. Management's best estimate of the value of the land was $100,000 and of the building $250,000. However, management told the accounting...
-
Using the data in the following table, predict the sign and magnitude of ÎH° for each of the following reactions. In each case, identify whether the reaction is expected to be endothermic...
-
At room temperature, molecules spend most of their time in lower energy conformations. In fact, there is a general tendency for any system to move toward lower energy. As another example, electrons...
-
At the end of the current month, Gil Frank prepared a trial balance for College App Services. The credit side of the trial balance exceeds the debit side by a significant amount. Gil has decided to...
-
What are the primary tenets of the Wagner Act?
-
What types of mortgages are available to borrowers?
-
What are the general provisions of the post-Sherman antitrust laws?
-
U.S. Economy Shrank 5% In the First Quarter The Commerce Department reported that gross domestic product fell at an annual rate of 5% in the first quarter. How does an unexpected decrease in the...
-
Analyze the frame in Figure P10.23. Compute the reactions and draw the shear and moment diagrams for members \(A B\) and \(B D\). Given: \(E I\) is constant. A w = 5 kips/ft 15- B D 12'
-
Find the length s and area A. Round answers to three decimal places. 2 ft Klem
-
Separate variables and use partial fractions to solve the initial value problems in Problems 18. Use either the exact solution or a computer-generated slope field to sketch the graphs of several...
-
Outline all steps in a malonic ester synthesis of each of the following: (a) pentanoic acid, (b) 2-methylpentanoic acid, (c) 4-methylpentanoic acid.
-
The antiepileptic drug valproic acid is 2-propylpentanoic acid (administered as the sodium salt). One commercial synthesis of valproic acid begins with ethyl cyanoacetate. The penultimate step of...
-
Show how you could employ enamines in syntheses of the following compounds: (a) (b) (c) (d) O C OEt
-
ABC had the following income statement for the period ending on December 31, 2020. Income Statement for the year ended 31/12/2020 Net Sales 120,000 Cost of Goods Sold 80,000 Gross Profit 40,000...
-
SERIE MONTO NOMINAL UNIDAD DE TASA DE PLAZO FINAL REAJUSTE DEL INTERSCUPN COLOCADO BONO VIGENTE A $100.000.000 Dlares 5,5% 01-01-2028 B $55.000.000 Dlares 3,4% 01-04-2032 C $45.000.000 Dlares 5,5%...
-
Cullumber Company's balance sheet at December 31, 2026 reports assets of $1068000 and liabilities of $737000. All of Cullumber's assets' book values approximate their fair value, except for land,...

Study smarter with the SolutionInn App


