Question: Using the data in the following table, predict the sign and magnitude of ÎH° for each of the following reactions. In each case, identify whether
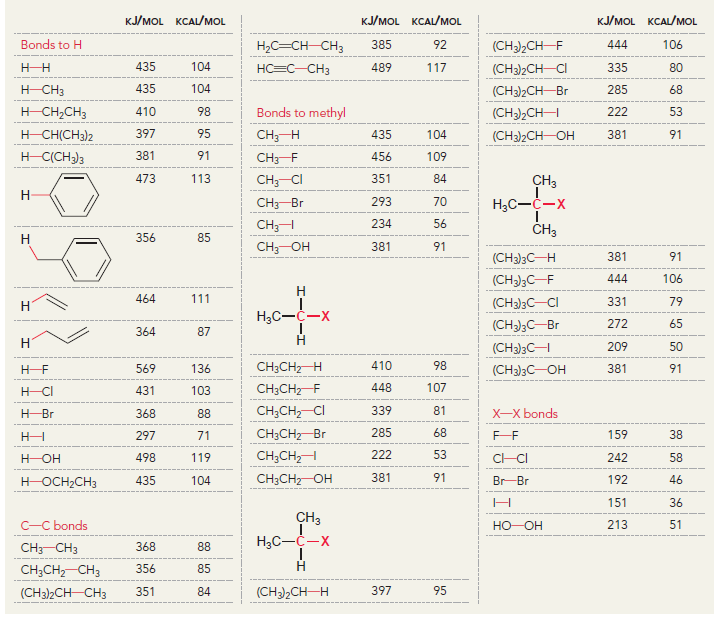
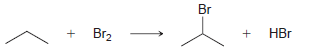
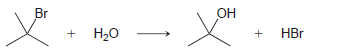
Using the data in the following table, predict the sign and magnitude of ΔH° for each of the following reactions. In each case, identify whether the reaction is expected to be endothermic or exothermic:
a.
b.

c.
d.

KJ/MOL KCAL/MOL ./OL KCAL/MOL J/OL KCAL/OL H (CH3)2CH F (CH3)2CH CI Bonds to H 385 92 444 106 435 104 C-- 489 117 335 80 H CH3 435 104 (CH3)2CH-Br 285 68 -CH Bonds to methyl 410 98 222 53 (CH3),CHH ICH)2 397 95 CHH 435 104 (CH3)2CH-OH 381 91 H) 381 91 456 109 CH3F CH3 473 113 351 84 CH; CI 293 70 CH3-Br 234 56 CH;H CH 356 85 C 381 91 (CH)3C 381 91 (CH)3C-F 444 106 111 464 (CH3)3C-CI 331 79 272 65 (CH 364 87 (CH)3C 209 50 CH3CH2 H 410 98 569 136 (CH)3 381 91 448 107 CH3CH2 F H-CI 431 103 CH;CH,-CI 339 81 X-X bonds HBr 368 88 CH3CH2 Br 285 68 159 38 297 71 222 53 CH;CH,H 498 119 C-CI 242 58 H-OH C CHz 381 91 435 192 46 HOCH2CH3 104 Br Br 151 36 CH C-C bonds 213 51 - H CH C 368 88 CH,CH CH 356 85 (CH)2CH H 351 397 95 84 (CH3)2CH CH3 Br Br, Br
Step by Step Solution
3.36 Rating (174 Votes )
There are 3 Steps involved in it
a Bonds Broken kJmol Bonds Formed kJmol H x CH CH 3 2 397 CH 3 2 CH x Br 285 Br x Br 192 H x Br 368 ... View full answer

Get step-by-step solutions from verified subject matter experts


