For most ketones, hydrate formation is unfavorable, because the equilibrium favors the ketone rather than the hydrate.
Question:
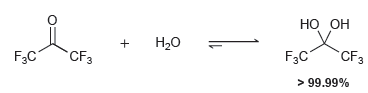
Transcribed Image Text:
НО Он Н.о F3C `CF3 F3C CF3 > 99.99%
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
The carbonyl group in hexafluoroacetone is flanked by two very pow...View the full answer

Answered By

Muhammad Salman Alvi
Well, I am a student of Electrical Engineeing from Information Technology University of Punjab. Just getting into my final year. I have always been good at doing Mathematics, Physics, hardware and technical subjects. Teaching profession requires a alot of responsibilities and challenges.
My teaching experience started as an home tutor a year ago. When I started teaching mathematics and physic subjects to an O Level student. He was about 14 years old. His name was Ibrahim and I used to teach him for about 2 hours daily. Teaching him required a lot of patience but I had to be polite with him. I used to give him a 5 min break after 1 hour session. He was quite weak in basic maths and calculation. He used to do quite a lot of mistakes in his homework which I gave him weekly. So I decided to teach him basics from scratch. He used to say that he got the concept even if he didn't. So I had to ask him again and again. I worked on his basics for a month and after that I started taking a weekly test sesions. After few months he started to improve gradually. Now after teaching him for about a year I can proudly say that he has improved alot. The most important thing was he managed to communicate all the difficullties he was facing. He was quite capable and patient. I had a sincere desire to help him reach to its full potential. So I managed to do that. We had a very good honest relationship of a student and a teacher. I loved teaching him as a tutor. Now having an experience of one year teaching I can read students quite well. I look forward to work as an online tutor who could help students in solving their all sort of difficulties, problems and queries.
4.90+
29+ Reviews
43+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The ÎG° for the equilibrium in Fig. P7.67ais 4.73 kJ mol-1 (t.13 kcal mol-1. (The equilibrium favors conformation A.) (a) Which behaves as if it is larger, methyl or phenyl (Ph)? iWhy is...
-
The equilibrium constant for hydrate formation from acetaldehyde (CH3CHO) is 1, and at equilibrium, there is an equal amount of acetaldehyde and its hydrate (CH3CH(OH)2) in solution. The equilibrium...
-
a. Would you expect hemiacetals to be stable in basic solutions? Explain your answer. b. Acetal formation must be catalyzed by an acid. Explain why it cannot be catalyzed by CH3O-. c. Can the rate of...
-
The accounting records for The Skate Shed, Inc., reflected the following amounts at the end of January 2018: Prepare The Skate Shed?s multistep income statement for the fiscal year ended January 31,...
-
PricewaterhouseCoopers is a Big Four Accounting firm that was facing high turnover in a key employee segment-senior associates. This is the second stage in a career ladder that starts at the entry...
-
See Table 2.5 showing financial statement data and stock price data for Mydeco Corp. a. What were Mydecos gross margins each year? b. Comparing Mydecos gross margin, EBIT margin, and net profit...
-
Farmer D. Jones has a crop of grapefruit that will be ready for harvest and sale as 150,000 pounds of grapefruit juice in 3 months. Jones is worried about possible price changes, so he is considering...
-
Suppose you are the victim of an identity thief who continues to use your identity and to ruin your credit rating after you have discovered the problem. What problems do you have in clearing your...
-
How do contingency factors, such as organizational size, industry dynamics, and institutional pressures, influence the optimal design of organizational structures and necessitate periodic...
-
In order to move a wrecked truck, two cables are attached at A and pulled by winches B and C as shown. Knowing that the tension in cable AC is 1.5 kips, determine the components of the force exerted...
-
Draw a mechanism for each of the following reactions: (a) (b) HO 1) EtMgBr 2) H20
-
Draw a plausible mechanism for each of the following transformations: (a) (b) (c) (d) Meo, OMe [H,SO4] excess MeOH -H20
-
A is the point (3, 4) and B is the point (5, 10). Find the equation of the perpendicular bisector of AB.
-
6.25 pts What is the future value at the end of year nine (9) of a $3000 initial investment today and two $1500 deposits at the end of year five (5) and at the end of year seven (7). The annual...
-
In an interest rate swap, what would be a typical range (order of magnitude) for the exposure (the amount that could be lost in case of a default of the counterparty) in percent of the principal? Why?
-
Explain how price expectations influence the level of interest rates.
-
If the Electric Potential (V) for a negative charge (-Q) at a distance 200 cm is equal to -11250 v. Calculate the electric potential energy if another negative charge Q = -2.42 mC is placed at the...
-
Find the electric poetintial when R = 3 cm and sigma 2 micro c \ m ^ 2 and x = 8 cm.
-
If one ball is selected at random, determine the odds against it containing a stripe. (Balls numbered 9 through 15 contain stripes.) Use the rack of 15 billiard balls shown. 1 15) (14 (13) (12 (11 10
-
On average there are four traffic accidents in a city during one hour of rush-hour traffic. Use the Poisson distribution to calculate the probability that in one such hour there arc (a) No accidents...
-
Consider a chemical species (either a molecule or an ion) in which a carbon atom forms three single bonds to three hydrogen atoms and in which the carbon atom possesses no other valence electrons....
-
Consider a chemical species like the one in the previous problem in which a carbon atom forms three single bonds to three hydrogen atoms, but in which the carbon atom possesses an unshared electron...
-
Consider another chemical species like the ones in the previous problems in which a carbon atom forms three single bonds to three hydrogen atoms but in which the carbon atom possesses a single...
-
A corporation issues 13 %, 15-year bonds with a par value of $570,000 and semiannual interest payments. On the issue date, the annual market rate for these bonds is 11%, which implies a selling price...
-
A production department reports the following conversion costs. Equivalent units of production for conversion total 436,000 units this period. Calculate the cost per equivalent unit of production for...
-
If you were asked whether a large university such as Tennessee or Michigan with a large seating capacity for their football stadiums should build a new football stadium, how would you respond and...

Study smarter with the SolutionInn App


