The following two compounds each exhibit two heteroatoms (one nitrogen atom and one oxygen atom). In compound
Question:
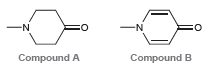
In compound A, the lone pair on the nitrogen atom is more likely to function as a base. However, in compound B, the lone pair on the oxygen atom is more likely to function as a base. Explain this difference.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





