The most stable conformation of 5-hydroxy-1,3-dioxane has the OH group in an axial position, rather than an
Question:
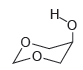
Transcribed Image Text:
Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
Although the OH group is in an ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Draw the most stable conformation of: (a) ethylcyclohexane (b) 3-isopropyl-1,1-dimethylcyclohexane (c) cis-1-tert-butyl-4-isopropylcyclohexane
-
The most stable conformation of trans-1,3-bis(1,1-dimethylethyl(cyclohexane is not a chair. What conformation would you predict for this molecule? Explain.
-
For most ketones, hydrate formation is unfavorable, because the equilibrium favors the ketone rather than the hydrate. However, the equilibrium for hydration of hexafluoroacetone favors formation of...
-
Use lHpitals rule to find the limit. 31 + 3 43 14t - t + 3 lim
-
You will work on how to receive and incorporate feedback. Specifically, you will use the feedback you received on your literature review draft
-
A three-legged wooden bar stool made out of solid Douglas fir has legs that are \(2.0 \mathrm{~cm}\) in diameter. When a \(75 \mathrm{~kg}\) man sits on the stool, by what percent does the length of...
-
For the simple linear regression model, show that the elements of the hat matrix are \[h_{i j}=\frac{1}{n}+\frac{\left(x_{i}-\bar{x} ight)\left(x_{j}-\bar{x} ight)}{S_{x x}} \text { and } h_{i...
-
Figure shows three rotating, uniform disks that are coupled by belts. One belt runs around the rims of disks A and C. Another belt runs around a central hub on disk A and the rim of disk B. The belts...
-
Presented below is information related to Larkspur Company. Cost Retail Beginning inventory $362,797 $286,000 Purchases ,370,000 2,145,000 Markups 95,100 Markup cancellations Markdowns Markdown...
-
Kent Company purchased 35 percent ownership of Lomm Company on January 1, 20X8, for $140,000. Lomm reported 20X8 net income of $80,000 and paid dividends of $20,000. At December 31, 20X8, Kent...
-
Consider the following chair conformation of bromocyclohexane: (a) Identify whether the bromine atom occupies an axial position or an equatorial position in the conformation above. (b) Draw a...
-
Draw both conformations for each of the following compounds: (a) (b) (c) (d) (e) (f) (g) (h) Br
-
Write the first five terms of the sequence for which (a) a 1 = 8 and d = 1/2; (b) a 1 = 8 and r = 1/2.
-
Explain the difference between a product and a brand.
-
Discuss the role and limitations of the external analysis phase of marketing planning.
-
Kaisers installs a new milkshake machine that increases labor productivity. If the price of a milkshake remains at $4, does Kaisers change the number of workers it hires? Explain. Kaisers Ice Cream...
-
What happens when people who might ordinarily come into contact with one another on their jobs no longer have that social contact? Several things may happen. For example, when employees do not see...
-
The Framers of the U.S. Constitution clearly wanted to limit the legislative power of the national government. They made this desire quite clear by enumerating the powers that Congress would possess...
-
Find the amplitude, period, and phase shift of function. Graph function. Show at least two periods. y = 2 cos
-
In Exercises evaluate the limit, using LHpitals Rule if necessary. lim 07x cos x X
-
Account for the following observations with mechanistic explanations. (a) At high concentration of ethoxide, the rate depends on both the allylic halide and ethoxide concentrations. (b) At low...
-
1-Chloro-3-methyl-2-butene undergoes hydrolysis in a mixture of water and dioxane at a rate that is more than a thousand times that of 1-chloro-2-butene. (a) What factor accounts for the difference...
-
Primary halides of the type ROCH2X apparently undergo SN1-type reactions, whereas most primary halides do not. Can you propose a resonance explanation for the ability of halides of the type ROCH2X to...
-
Speedy Auto Repairs uses a job-order costing system. The company's direct materials consist of replacement parts installed in customer vehicles, and its direct labor consists of the mechanics' hourly...
-
3. A, B and C are partners sharing profits and losses equally. The balance sheet at 31st December 2020 is as follows. Assets Liabilities Amount Amount (RO) (RO) Creditors 5,000 Cash at Bank 3,000...
-
For each of the following program fragments, give an analysis of the running time. 1) sum = 0; for (int i = n; i > 0; i--) for (int j = 0; j

Study smarter with the SolutionInn App


