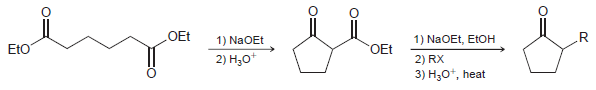
The product of a Dieckmann cyclization can undergo alkylation, hydrolysis, and decarboxylation. This sequence represents an efficient
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





