When 2 moles of benzaldehyde are treated with sodium hydroxide, a reaction occurs in which 1 mole
Question:
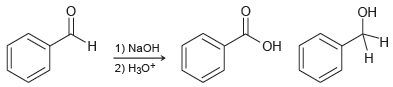
This reaction, called the Cannizzaro reaction, is believed to occur via the following mechanism: A hydroxide ion serves as a nucleophile to attack the carbonyl group of benzaldehyde, generating a tetrahedral intermediate. This tetrahedral intermediate then functions as a hydride reducing agent by delivering a hydride ion to another molecule of benzaldehyde. In this way, one molecule is reduced while the other is oxidized.
(a) Using the explanation above, draw the mechanism of the Cannizzaro reaction.
(b) What is the function of H3O+ in the second step?
(c) Water alone is not sufficient to accomplish the function of the second step. Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





