Question: (a) What does a positive entropy change in a closed system imply about the change in the number of basic states? (b) How does Eq.19.8
(a) What does a positive entropy change in a closed system imply about the change in the number of basic states?
(b) How does Eq.19.8 show that a gas expands if given more room?
(c) How does it show that the gas does not contract spontaneously into a subvolume?
Equation
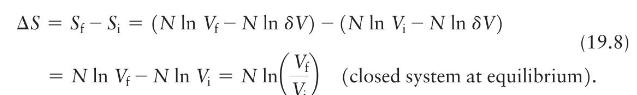
ASS S = (N In V - N In 8V) - (N In V - N In 8V) (19.8) = N In V-N In V = N In In M (closed system at equilibrium).
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


