Question: Explain why the changes in entropy in Example 19.10 do not depend on the volume (V) of the box. Data from Example 19.10 The box
Explain why the changes in entropy in Example 19.10 do not depend on the volume \(V\) of the box.
Data from Example 19.10
The box in Figure 19.19 contains seven gas particles in compartment \(\mathrm{A}\) and five in compartment \(\mathrm{B}\), and the partition separating the compartments is free to move. Let the volume of the box be \(V\) and the initial ratio of the compartment volumes be \(V_{\mathrm{B}, \mathrm{i}} / V_{\mathrm{A}, \mathrm{i}}=3\). What is the change in the entropy of this gas as it evolves from this macrostate to equilibrium?
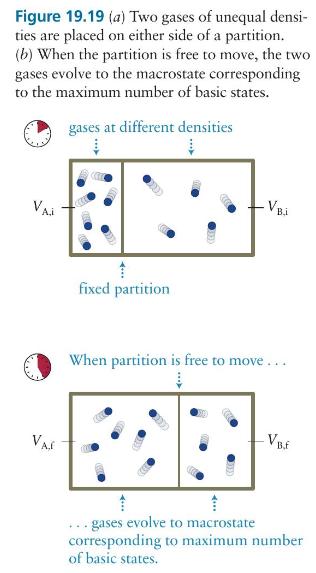
Figure 19.19 (a) Two gases of unequal densi- ties are placed on either side of a partition. (b) When the partition is free to move, the two gases evolve to the macrostate corresponding to the maximum number of basic states. gases at different densities Vai fixed partition VB.i When partition is free to move... VA.E 1 1 gases evolve to macrostate VB.F corresponding to maximum number of basic states.
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
Even though entropy depends on volume the entrop... View full answer

Get step-by-step solutions from verified subject matter experts


