Question: You need a liquid electrical conductor for a project you are working on, and as one possibility you try seawater. You know the number density
You need a liquid electrical conductor for a project you are working on, and as one possibility you try seawater. You know the number density of the charge carriers and the average time interval between collisions. Your preliminary calculations using Eq. 31. 9 and the mass and charge of an electron give you a theoretical conductivity, \(\sigma_{\text {theory }}\). When testing the seawater, though, you obtain a conductivity less than two thousand times smaller than \(\sigma_{\text {theory }}\left(\sigma_{\text {test }})\). You struggle to understand this discrepancy.
Data from Eq. 31. 9
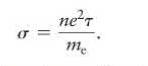
= neT me
Step by Step Solution
3.35 Rating (158 Votes )
There are 3 Steps involved in it
The discrepancy between the theoretical and measured conductivity of seawater arises due to incorrec... View full answer

Get step-by-step solutions from verified subject matter experts


