1.10 moles of N 2 at 20.5°C and 6.20 bar undergoes a transformation to the state described...
Question:
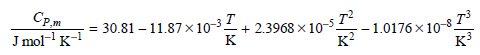
Transcribed Image Text:
Сри Jmol-1 к-1 + 2.3968 x 10- г? -1.0176 х 10-3. 30.81-11.87х10 3. -3T к? кз к
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
Cpm Jmol K 3081 118710 23968 x 105 T Pf ASnR In n P T 48815 ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard entropy of Pb(s) at 298.15 K is 64.80 J K -1 mol - 1 . Assume that the heat capacity of Pb(s) is given by The melting point is 327.4C and the heat of fusion under these conditions is...
-
A 2.5 x 2.5 m steel sheet 1.5 mm thick is removed from an annealing oven at a uniform temperature of 425?C and placed in a large room at 20?C in a horizontal position.(a) Calculate the rate of heat...
-
A plastic tube of 7.6-cm ID and 1.27 cm wall thickness having a thermal conductivity of 1.7 W/(m K), a density of 2400 kg/m3, and a specific heat of 1675 J/(kg K) is cooled from an initial...
-
Mr. Silkwallah established the Fashion Clothing Company (FCC) to market designer clothes. The business was to get designer clothes produced by tailors, exclusively for FCC. FCC provides the following...
-
What behaviors do effective leaders exhibit?
-
A eukaryotic protein-encoding gene contains two introns and three exons: exon 1intron 1exon 2intron 2exon 3. The 5 splice site at the boundary between exon 2 and intron 2 has been eliminated by a...
-
Model equations similar to those for potential flow arise in flow in porous media, which has a wide variety of applications, e.g., in groundwater treatment, water-purity remediation, filtration, flow...
-
If the risk-free rate is 6% and the expected rate of return on the market portfolio is 13%, is a security with a beta of 1.25 and an expected rate of return of 16% overpriced or underpriced?
-
A large 5-kg marble moves at a speed of 20 m/s. It collides with a second marble whose mass is 10-kg, moving at 10 m/s in the same direction. After the collision, the 5-kg marble continues with a...
-
For the circuit shown in Fig. 18.31, find (a) The cur-rent in each resistor, (b) The voltage across each resistor, and (c) The total power delivered. R2 " 20 R:" 20
-
What is design capacity? Effective capacity? Best operating level?
-
Propose a plausible synthesis for the following transformation. CH3 CH3 CH3
-
List some benefits of enterprise chatbots.
-
State the contrapositive and converse. If \(m^{2}\) is divisible by 3 , then \(m\) is divisible by 3 .
-
State the negation. \(x\) is a real number and \(y\) is an integer.
-
What is the difference between a diagram and a model?
-
Give an example of some knowledge that you possess. What is its purpose?
-
Which is the negation of the statement, "The car and the shirt are both blue"? (a) Neither the car nor the shirt is blue. (b) The car is not blue and/or the shirt is not blue.
-
In problem, find the exact values of the six trigonometric functions of the given angle. If any are not defined, say not defined. Do not use a calculator. -13/6
-
Prairie Outfitters, Inc., a retailer, accepts paymnent through credit cards. During August, credit card sales amounted to $12,000. The processor charges a 3% fee. Assuming that the credit card...
-
Find the three cube roots of 3.000 2.000i .
-
Find the four fourth roots of 3.000i.
-
Find the real and imaginary parts of (3.00 + i) 3 + (6.00 + 5.00i) 2 . Find z .
-
S&J Catering is a small catering business operating in Western Sydney. Business partners, Jack and Simon, established the business a year ago. To start up the business, the partners contributed...
-
In 20X1, Lee was hurt in the course of his employment with Foster Farms. In 20X1, Lee was paid the following amounts from Foster Farms' worker's compensation insurance company: $30,000 for a physical...
-
Briefly describe tax administrative matters including: Tax collection and withholding mechanisms (PAYG withholding, PAYG instalments).

Study smarter with the SolutionInn App


