A liquid mixture of benzene and toluene is to be separated in a continuous single-stage equilibrium flash
Question:
A liquid mixture of benzene and toluene is to be separated in a continuous single-stage equilibrium flash tank.
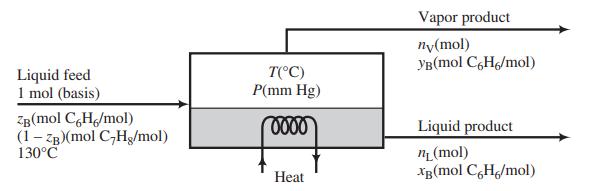
The pressure in the unit may be adjusted to any desired value, and the heat input may similarly be adjusted to vary the temperature at which the separation is conducted. The vapor and liquid product streams both emerge at the temperature T(°C) and pressure P(mm Hg) maintained in the vessel. Assume that the vapor pressures of benzene and toluene are given by the Antoine equation, APEx; that Raoult’s law—Equation 6.4-1—applies; and that the enthalpies of benzene and toluene liquid and vapor are linear functions of temperature. Specific enthalpies at two temperatures are given here for each substance in each phase.

Equation 6.4-1
![]()
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard




