At 30 km above the Earths surface (roughly in the middle of the stratosphere), the pressure is
Question:
a. The number of collisions a single gas particle undergoes in this region of the stratosphere in 1.0 s
b. The total number of particles collisions that occur in 1.0 s
c. The mean free path of a gas particle in this region of the stratosphere Using the ideal gas law, the temperature at 30 km can be determined as follows:
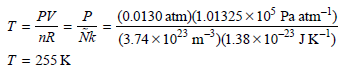
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





