At sufficiently high temperatures, the van der Waals equation has the form P L RT>(V m -
Question:
Figure 1.10
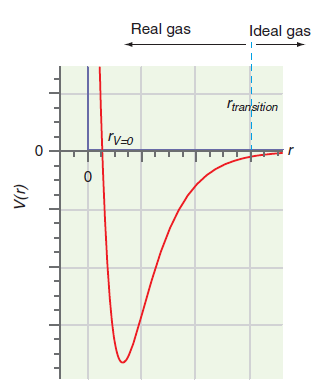
Transcribed Image Text:
Ideal gas Real gas Itrarlsition (1)A
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 46% (15 reviews)
At high temperatures the energy of the molecule is lar...View the full answer

Answered By

Zablon Gicharu
I am an educator who possesses the requisite skills and knowledge due to interacting with students for an extended period. I provide solutions to various problems in step-by-step explanations, a well-thought approach and an understandable breakdown. My goal is to impart more straightforward methodologies and understanding to students for more remarkable achievements.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
For carbon dioxide gas the constants in the van der Waals equation are = 0.364 J m 3 /mol 2 and (a) If 1.00 mol of gas at 350 K is confined to a volume of find the pressure of the gas using the...
-
In Sample Exercise 10.16, we found that one mole of Cl2 confined to 22.41 L at 0oC deviated slightly from ideal behavior. Calculate the pressure exerted by 1.00 mol Cl2 confined to a smaller volume,...
-
The parameter a in the van der Waals equation is greater for H 2 O than for He. What does this say about the difference in the form of the potential function in Figure 1.10 for the two gases? Figure...
-
A mortgage loan officer uses math on a continual basis during the mortgage lending process. When a prospective borrower applies for a loan, many calculations are made: debt-to-income- ratio,...
-
The following transactions have also occurred at Fitzgerald. 1. Options were granted on July 1, 2013, to purchase 200,000 shares at $15 per share. Although no options were exercised during fiscal...
-
Predicting Delayed Flights. The file FlightDelays.csv contains information on all commercial flights departing the Washington, DC area and arriving at New York during January 2004. For each flight,...
-
Discuss the pros and cons of using social networks to recruit top talent.
-
1. Use ethical reasoning to evaluate the actions of Shell management in this case with respect to accounting for and disclosing information about proved reserves. 2. In chapter 7 we discussed...
-
If the Consumer Price Index rose from 250 to 258 in one year, what was the approximate annual inflation rate for that year? An effective financial plan must be adaptable to changing circumstances....
-
a. What would happen if octane was added to a solution of sodium hydroxide? b. Explain your answer to part a.
-
Define the seven aspects of customer service.
-
What are some uses for hydrogen fuel cells?
-
Given the following information, determine the taxable portion and return of capital in each situation, as well as accumulated E&P on January 1, 2020. 2019 Current E&P a. $75,000 b. 75,000 75,000...
-
how would you evaluate your hiring experience? what would you change about the hiring process if you were responsible for hiring and retaining employees
-
Deferred compensation is another practice that employers may choose to adopt in certain circumstances. Describe three benefits an employer might experience from using deferred compensation.
-
Give detailed explanation of STEM ( Two paragraphs with references) Explain the importance of STEM education with the help of technology?
-
Explain internal and external resourcing. A. Identify and explain internal resourcing. B. Identify and explain external resourcing
-
in regards to disney world why should an organization be concerned about labor relations? Contrast the style of labor unions in the U.S. to that found in another country? Also, briefly discuss ways...
-
A suitable restriction on the domain of the function f(x) = (x - 1) 2 to make it one-to-one would be ______.
-
Is times interest earned meaningful for utilities? Why or why not?
-
For PbI2(s) = 0Pb+(aq) + 2 r(aq), K = 1.4 X 10-8 at 25C and the standard Gibbs energy of formation ofPbI2(s) is -173.64 k] mol ". Calculate the standard Gibbs energy of formation of PbI2 (aq).
-
Write the cell reaction and electrode half-reactions and calculate the standard emf of each the following cells: (a) Ptl C12 (g) I HCl (aq) 11 K, Cr04 (aq) IAg, Cr04(s) IAg (b) Pt 1 Fe3+(aq),Fe2+(aq)...
-
Devise cells in which the following are the reactions and calculate the standard emf in each case: (a) 2 Na(s) + 2 H20 (l) --7 2 NaOH (aq) + H2 (g) (b) H2 (g) + I2 (g) --72 HI (aq) (c) H30+ (aq) + OW...
-
What is Fibonacci heap? Explain CONSOLIDATE operation with suitable example for Fibonacci heap ?
-
Discuss the impact of global supply chain disruptions, exacerbated by events like the COVID-19 pandemic, on inventory management strategies and market resilience across various industries?
-
A pharmaceutical retailer decided to host a website for home delivery of medicines according to user orders. The web application is deployed on a single Amazon EC2 instances. within a few months, the...

Study smarter with the SolutionInn App


