Question: Consider the comparison made between accurate results and those based on calculations using the van der Waals and RedlichKwong equations of state in Figures 7.1
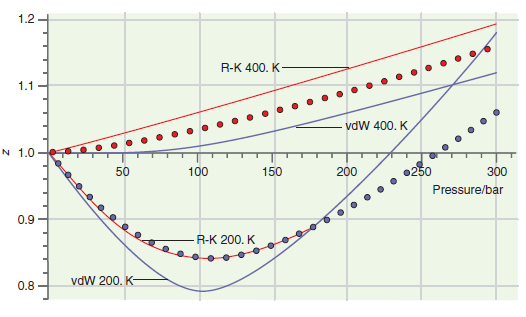
Consider the comparison made between accurate results and those based on calculations using the van der Waals and Redlich€“Kwong equations of state in Figures 7.1 and 7.5. Is it clear that one of these equations of state is better than the other under all conditions?
Figure 7.1
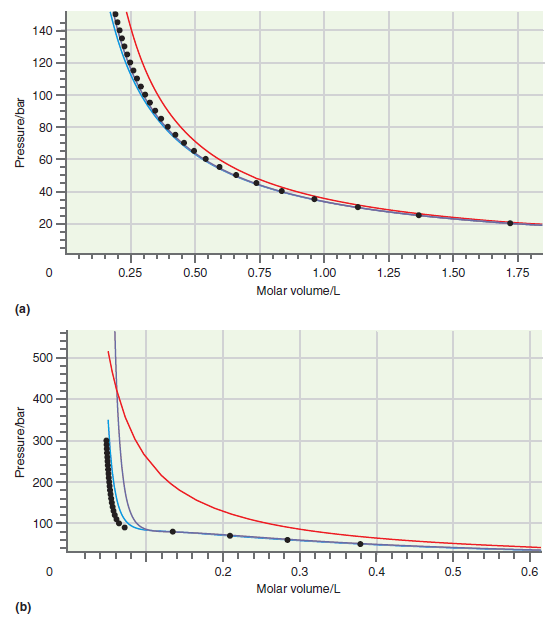
Figure 7.5
140 120 100 80 40 20 0.25 0.75 0.50 1.00 1.25 1.50 1.75 Molar volume/L (a) 500 400 300 200 100 0.2 0.3 0.4 0.5 0.6 Molar volume/L (b) Pressure/bar Pressure/bar 1.2 R-K 400. K 1.1 vdW 400. K N 1.0 100 150 200 250 300 Pressure/bar 0.9 R-K 200. K vdW 200. K- 0.8
Step by Step Solution
3.29 Rating (164 Votes )
There are 3 Steps involved in it
The RedlichKwong gives more ac... View full answer

Get step-by-step solutions from verified subject matter experts


