Consider the equilibrium CO(g) + H2O(g) ???? CO2(g) + H2(g). At 1150. K, the composition of the
Question:
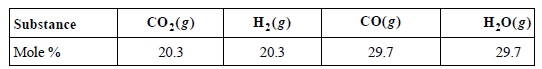
a. Calculate KP and ΔGoR at 1150. K.
b. Given the answer to part (a), use the ΔHof of the reaction species to calculate ΔGoR at 298.15 K. Assume that ΔHR° is independent of temperature.
Transcribed Image Text:
CO(g) co:(g) H;(g) H,0(g) Substance 20.3 29.7 Mole % 20.3 29.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (14 reviews)
a COg H 2 OgCO 2 g H 2 g For each compo...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A chamber initially contains a gaseous mixture consisting of 4 kmol of CO2, 8 kmol of CO and 2 kmol of H2. Assume an equilibrium mixture formed consists of CO2, CO, H2O, H2 and O2 at 2600 K and 100...
-
At 25 C, Kp < 1 10-31 for the reaction N2(g) + O2(g) 2NO(g) a. Calculate the concentration of NO (in molecules/ cm3) that can exist in equilibrium in air at 25oC. In air PN2 = 0.8 atm and PO2 = 0.2...
-
Consider the water-gas-shift reaction: H2(g) + CO2(g) H2O(g) + CO(g) At high temperatures and low to moderate pressures the reacting species form an ideal- gas mixture. By Eq. (11.27): When the Gibbs...
-
Show that plane stress displacements for the Flamant problem in Section 8.4.7 under only tangential force X are given by: Data from section 8.4.7 My (1 v) - - -0 sin (1 v)X, -0 cost + 2X, 20 log r...
-
List and explain the stages in the product life cycle. How can a small firm extend its product's life?
-
What is a haplotype? a. A species with one set of chromosomes b. A cell with one set of chromosomes c. The linkage of alleles or molecular markers on a chromosome d. All of the above describe a...
-
Diet Cola and Weight Gain in Humans A study found that American senior citizens who report drinking diet soda regularly experience a greater increase in weight and waist circumference than those who...
-
Abbey Park was organized on April 1, 2016, by Trudy Crawford. Trudy is a good manager but a poor accountant. From the trial balance prepared by a part-time bookkeeper Trudy prepared the following...
-
Steven's Cat Math: This weekend I observed a cat, known as Fluffy, running across a branch and attempting to jump up to another tree. Unfortunately, Fluffy missed the other tree and fell to the...
-
The sample function of a Gaussian process of zero mean and unit variance is uniformly sampled and then applied to a uniform quantizer having the input-output amplitude characteristic shown in Figure....
-
Calculate K P at 600.K for the reaction N 2 O 4 (l) 2NO 2 (g) assuming that H o R is constant over the interval 298 725 K.
-
When 2,4-dibromo-3-methyltoluene is treated with bromine in the presence of iron (Fe), a compound with molecular formula C8H7Br3 is obtained. Identify the structure of this product.
-
Is there evidence that mean heart rate is higher in male ICU patients than in female ICU patients? This dataset, stored in ICU Admissions, contains information about a sample of patients admitted to...
-
Find the sum of the first 50 terms of the arithmetic sequence whose first term is -15 and whose common difference is 5 .
-
Suppose someone tells you she has traced her family tree back 10 generations. What is the minimum number of people on her family tree if there were no intermarriages?
-
a. Find the first three terms of the sequences whose nth terms are given. b. Classify the sequence as arithmetic (give d), geometric (give r), both, or neither. \(s_{n}=-5 \)
-
In Problems 47-56, decide whether you would use a permutation, a combination, or neither. Next, write the solution using permutation notation or combination notation, if possible, and, finally,...
-
Evaluate each expression in Problems 3-32. \(\left(\begin{array}{l}9 \\ 0\end{array}ight)\)
-
Determine the amplitude and period of each function without graphing. y = -3 cos(3x)
-
In July 2013, cnet.com listed the battery life (in hours) and luminous intensity (i. e., screen brightness, in cd/m2) for a sample of tablet computers. We want to know if screen brightness is...
-
Find the real root of the equation x 2 e x = 0.
-
Find the root of the equation x 2.00 sin (x) = 0.
-
Find two positive roots of the equation ln (x) 0.200x = 0.
-
Find the integrating factor for the given 1-order linear non-homogeneous ordinary differential equation. Do not solve the ordinary differential equation. y xdx-xdy-x+dx + dx
-
[Bush] Consider the following snippet of code. (Assume that input strings, including null terminator, will always fit within the size 255 array.) char* to_upper_case(char* original) { char...
-
50. Show that if f(x) = a,x" + a-1x+...+x+ ao, a,..., a-1, and a,, are real numbers and where 0, then f(x) is O(x"). an # Big-O, big-Theta, and big-Omega notation can be extended to functions in more...

Study smarter with the SolutionInn App


