Formaldehyde is produced commercially by the catalytic oxidation of methanol. In a side reaction, methanol is oxidized
Question:
Formaldehyde is produced commercially by the catalytic oxidation of methanol. In a side reaction, methanol is oxidized to CO2.
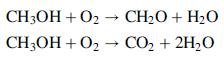
A mixture containing 55.6 mole% methanol and the balance oxygen enters a reactor at 350°C and 1 atm at a rate of 4:60 × 104 L/s. The reaction products emerge at the same temperature and pressure at a rate of 6:26 × 104 L/s. An analysis of the products yields a molar composition of 36.7% CH2O, 4.1% CO2, 14.3% O2, and 44.9% H2O. The required reactor cooling rate is calculated to be 1:05 × 105 kW.
(a) Is the calculated cooling rate correct for the given stream data?
(b) The stream data cannot be correct. Prove it.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





