Question: Given the data in Table 4.3 and the data tables, calculate the bond enthalpy and energy of the following: a. The CH bond in CH
a. The C€”H bond in CH4
b. The C€”C single bond in C2H6
c. The C£C double bond in C2H4
Use your result from part (a) to solve parts (b) and (c).
Table 4.3
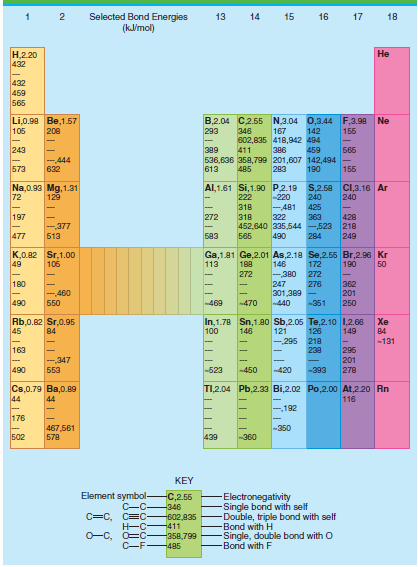
Selected Bond Energies (klmol) 13 14 15 16 17 18 .220 432 432 459 565 B,2.04 C2.55 N,3.04 0,3.44 F,3.98 Ne Li,0.90 Be,1.57 105 208 293 346 167 142 155 602,835 418,942 494 243 65 389 411 386 459 -444 536,636 358,799 201,607 142,494 155 573 632 613 485 283 190 Al,1.61 Si,1.90 P.2.19 S,2.58 C,3.16 Ar Na,0.93 Mg, 1.31 72 129 222 -220 240 240 -481 322 363 452,640 335,544 -523 218 318 425 197 272 318 428 -377 477 583 249 513 565 490 284 K,0.82 Sr,1.00 49 Ga,1.81 Ge,2.01 As,2.18 Se,2.55 Br,2.96 Kr 146 106 113 188 172 190 50 272 -380 272 180 247 362 201 250 276 301,389 - -440 -460 490 50 -469 470 -351 In,1.78 Sn,1.80 Sb,2.05 Te,2.10 1,2.66 121 -295 Rb,0.82 Sr,0.95 45 Xe 100 149 84 146 126 84 218 238 -131 163 296 201 278 -,347 553 490 -523 -420 450 -393 TI,2.04 Pb,2.33 Bi,2.02 Po,2.00o At,220 Rn Cs,0.79 Ba,0.89 44 116 44 192 176 -350 467,561 578 502 439 -360 KEY Element symbol- C-C C=C, C=C Electronegativity Single bond with self Double, triple bond with self Bond with H Single, double bond with O -Bond with F C,2.55 -346 602,835 -411 O-C, O=C- -358,799 C-F485
Step by Step Solution
3.36 Rating (174 Votes )
There are 3 Steps involved in it
a CH 4 g Cg 4Hg H o R 4H o f H g H o f C g H o f CH 4 g 4 2180 kJ mol 1 7167 kJ mol 1 746 kJ mol 1 1... View full answer

Get step-by-step solutions from verified subject matter experts


