Question: Solve the given problems by showing the complete working solution Exercises 33 Which of the following is equivalent to the bond enthalpy of the carbonoye
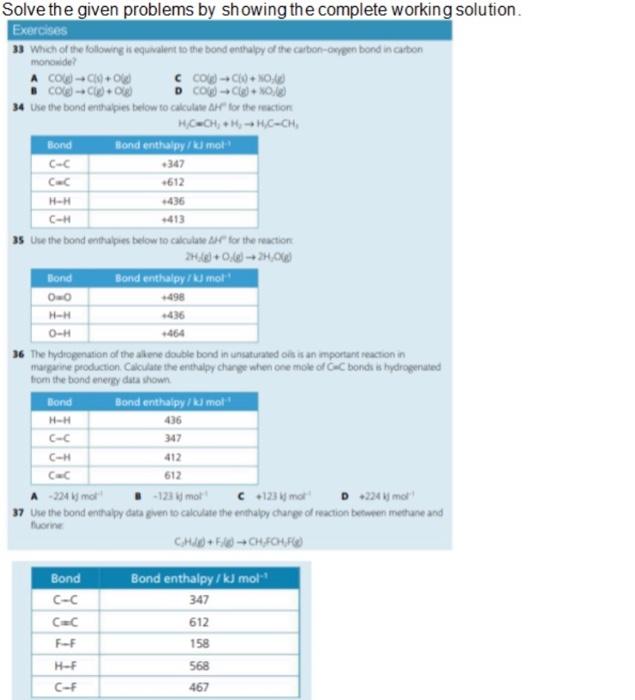
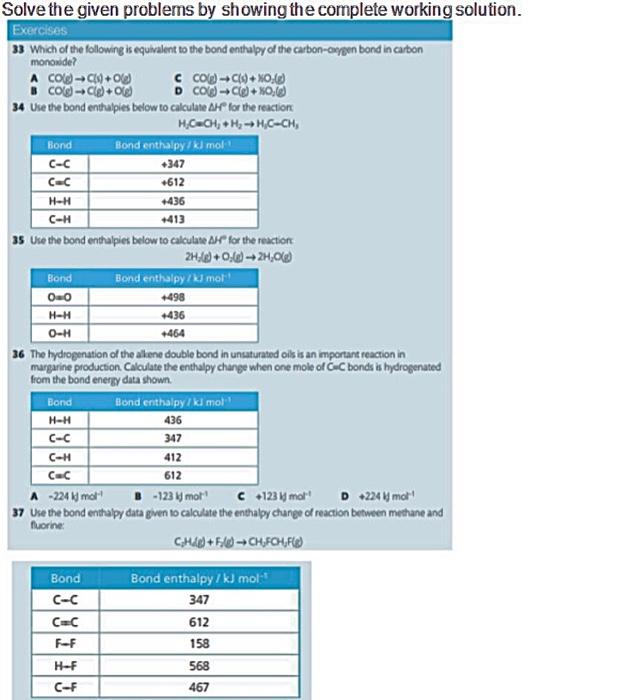
Solve the given problems by showing the complete working solution Exercises 33 Which of the following is equivalent to the bond enthalpy of the carbonoye bond in carbon monoxide A COCO c cog-NO . CO-Og D COC+) 34 Use the bond enthupies below to calculated for the reaction H CH + H - HC-CH, Bond Bond enthalpy / tumot C-C -347 CE -612 H-H 436 CH 35 Use the bond enthalpies below to calculate for the reaction 20:48 +24,06 Bond Bond enthalpy mo! OO -498 HH -436 0-# 464 36 The hydrogenation of the aliene double bond in unsaturatedols is an mportant reaction in margarine production Calculate the enthalpy change when one mole of GC bonds is hydrogenated from the bond energy data shown Bond Bond enthalpy / kl mot H-H 436 347 412 612 CE A 224 mo 123mol c123mor D 224 mot 37 Use the bond enthalpy data pen to calculate the enthalpy change of reaction between methane and turne CHICHFOUR Bond C-c CC F-F Bond enthalpy / kJ mol 347 612 158 568 467 H-F C-F Solve the given problems by showing the complete working solution. Exercises 33 Which of the following is equivalent to the bond enthalpy of the carbon-cwypen bond in carbon monoxide? A colg-C/4+0) C CO(g) +19+ XO() B Cog) -C+08 D Cod -C(+0,6) 34 Use the bond enthalples below to calculate Al for the reaction H=CH+ H - HC-CH, Bond Bond enthalpykl mol C-C 347 CEC +612 H-H +436 1413 35 Use the bond enthalpies below to calculate for the reaction 2H_() +0:0) -24,068 Bond Bond enthalpy / kl mol 00 498 +436 0-H +464 36 The hydrogenation of the alkene double bond in unsaturated oils is an important reaction in margarine production. Calculate the enthalpy change when one mole of C bonds is hydrogenated from the bond energy data shown Bond Bond enthalpykl mol H-H 436 3 C-H H-H C-C 347 C-H 412 C C 612 A -224 molt B-1231 more C +1234 molt D 2246 mol 37 Use the bond enthalpy data gwen to calculate the enthalpy change of reaction between methane and fuorine CH+50) - CHFCH,FB) Bond C-C Bond enthalpy / kJ mol 347 612 158 568 467 F-F H-F C-F
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


