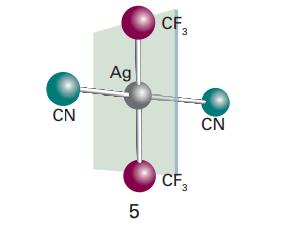
In the square-planar complex anion [trans-Ag(CF 3 ) 2 (CN) 2 ], the AgeCN groups are collinear.
Question:
In the square-planar complex anion [trans-Ag(CF3)2(CN)2]−, the AgeCN groups are collinear.
(a) Assume free rotation of the CF3 groups (that is, disregarding the AgCF and AgCH angles) and name the point group of this complex ion.
(b) Now suppose the CF3 groups cannot rotate freely (because the ion was in a solid, for example). Structure (5) shows a plane which bisects the NCeAgeCN axis and is perpendicular to it. Name the point group of the complex if each CF3 group has a CF bond in that plane (so the CF3 groups do not point to either CN group preferentially) and the CF3 groups are (i) staggered, (ii) eclipsed.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:





