Question: In this problem, you calculate the error in assuming that ÎH o R is independent of T for the reaction 2CuO(s) 2Cu(s) + O 2
In this problem, you calculate the error in assuming that ΔHoRis independent of T for the reaction
2CuO(s) ‡‹ 2Cu(s) + O2(g).
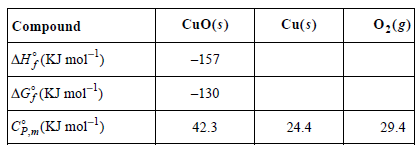
The following data are given at 25°C:
a. From Equation (6.65),
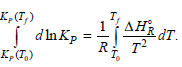
To a good approximation, we can assume that the heat capacities are independent of temperature over a limited range in temperature, giving ΔHoR (T) = ΔHoR (T0) + ΔCP(T ˆ’ T0), where ˆ†CP
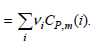
By integrating Equation (6.65), show that
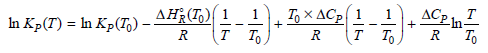
b. Using the result from part (a), calculate the equilibrium pressure of oxygen over copper and CuO(s) at 1275 K. How is this value related to Kp for the reaction 2CuO(s) ‡‹ 2Cu(s) + O2(g)?
c. What value would you obtain if you assumed that ΔHoR were constant at its value for 298.15 K up to 1275 K?
Cu(s) CuO(s) 0,(g) Compound AH;(KJ mol1) AG; (KJ mol") -157 -130 |CE,m(KJ mol-1) 42.3 24.4 29.4 T, 1 AHR K,(T,) dIn Kp | dnkp K,(T,) RdT. R T?
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it
a b 2CuOs 2Cus O 2 g In c This is equivalent to setting C P 0 Ne... View full answer

Get step-by-step solutions from verified subject matter experts


