Question: In this problem, you will use the variational method to find the optimal 1s wave function for the hydrogen atom starting from the trial function
In this problem, you will use the variational method to find the optimal 1s wave function for the hydrogen atom starting from the trial function Φ(r) = eˆ’αrwith α as the variational parameter. You will minimize
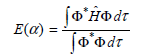
with respect to α.
a. Show that
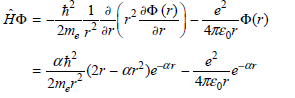
b. Obtain the result
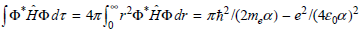
Using the standard integrals in the Math Supplement (Appendix A)
c. Show that
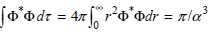
Using the standard integrals in the Math Supplement.
d. You now have the result E (α) = h2α2 / (2me) €“ e2α / (4πε0). Minimize this function with respect to α and obtain the optimal value of α
e. Is E(αoptimal) equal to or greater than the true energy? Why?
('r E(@) ) 2 () -(?) 4" %3D 2. 2, r- r r an? 2m,r z(2r ar?)ear 4EOr
Step by Step Solution
3.46 Rating (166 Votes )
There are 3 Steps involved in it
a We start with Using these result b c ... View full answer

Get step-by-step solutions from verified subject matter experts


