Question: The wave function for a hydrogen atom in the 2s state is(a) Verify that this function is normalized.(b) In the Bohr model, the distance between
The wave function for a hydrogen atom in the 2s state is(a) Verify that this function is normalized.(b) In the Bohr model, the distance between the electron and the nucleus in the n = 2 state is exactly 4a. Calculate the probability that an electron in the 2s state will be found at a distance less than 4a from thenucleus.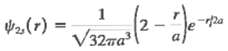
1 370/(2-7) e-72 -pa
Step by Step Solution
3.55 Rating (166 Votes )
There are 3 Steps involved in it
a Since the given r is real r lyf r The probability density wil... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
P-M-P-A-S (41).docx
120 KBs Word File


