Nitric acid is a chemical intermediate primarily used in the synthesis of ammonium nitrate, which is used
Question:
Nitric acid is a chemical intermediate primarily used in the synthesis of ammonium nitrate, which is used in the manufacture of fertilizers. The acid also is important in the production of other nitrates and in the separation of metals from ores.
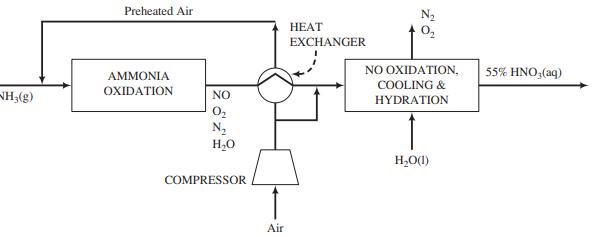
Nitric acid may be produced by oxidizing ammonia to nitric oxide over a platinum–rhodium catalyst, then oxidizing the nitric oxide to nitrogen dioxide in a separate unit where it is absorbed in water to form an aqueous solution of nitric acid.
The reaction sequence is as follows:
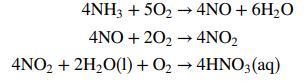
where, unless otherwise specified, the species are gases. A side reaction in which ammonia is oxidized to form nitrogen and water can lower product yield:
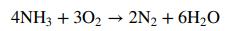
Ammonia vapor produced by vaporizing pure liquid ammonia at 820 kPa absolute is mixed with air, and the combined stream enters the ammonia oxidation unit. Air at 30°C, 1 atm absolute, and 50% relative humidity is compressed and fed to the process. A fraction of the air is sent to the cooling and hydration units, while the remainder is passed through a heat exchanger and mixed with the ammonia. The total oxygen fed to the process is the amount stoichiometrically required to convert all of the ammonia to HNO3 , while the fraction sent to the ammonia oxidizer corresponds to the stoichiometric amount required to convert ammonia to NO.
The ammonia reacts completely in the oxidizer, with 97% forming NO and the rest forming N2. Only a negligible amount of NO2 is formed in the oxidizer. However, the gas leaving the oxidizer is subjected to a series of cooling and hydration steps in which the NO is completely oxidized to NO2, which in turn combines with water (some of which is present in the gas from the oxidizer and the rest is added) to form a 55 wt% aqueous solution of nitric acid. The product gas from the process may be taken to contain only N2 and O2.
(a) Taking a basis of 100 kmol of ammonia fed to the process, calculate (i) the volumes (m3) of the ammonia vapor and air fed to the process using the compressibility-factor equation of state; (ii) the amount (kmol) and composition (in mole fractions) of the gas leaving the oxidation unit; (iii) the required volume of liquid water (m3) that must be fed to the cooling and hydration units; and (iv) the fraction of the air fed to the ammonia oxidizer.
(b) Scale the results from Part (a) to a new basis of 100 metric tons per hour of 55% nitric acid solution.
Exploratory Exercises—Research and Discover
(c) Nitrogen oxides (collectively referred to as NOx) are a category of pollutants that are formed in many ways, including processes like that described in this problem. List the annual emission rates of the three largest sources of NOx emissions in your home region. What are the effects of exposure to excessive concentrations of NOx?
(d) A platinum–rhodium catalyst is used in ammonia oxidation. Explain the function of the catalyst, describe its structure, and explain the relationship of the structure to the function.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard




