Nitrogen dioxide reacts bimolecularly in the gas phase to give 2 NO + O 2 . The
Question:
Nitrogen dioxide reacts bimolecularly in the gas phase to give 2 NO + O2. The temperature dependence of the second-order rate constant for the rate law d[P]/dt = k[NO2]2 is given below. What are the P factor and the reactive cross-section for the reaction?
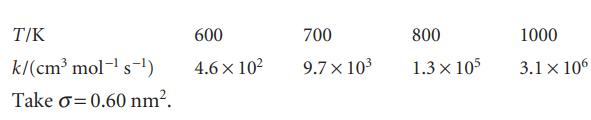
Transcribed Image Text:
T/K k/(cm³ mol-¹ s-¹) Take o=0.60 nm². 600 4.6 x 10² 700 9.7 x 10³ 800 1.3 x 105 1000 3.1 x 106
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The P factor include Reactive cross section for the reaction can be calcul...View the full answer

Answered By

James Warinda
Hi! I’m James Otieno and I'm an experienced professional online tutor with countless hours of success in tutoring many subjects in different disciplines. Specifically, I have handled general management and general business as a tutor in Chegg, Help in Homework and Trans tutor accounts.
I believe that my experience has made me the perfect tutor for students of all ages, so I'm confident I can help you too with finding the solution to your problems. In addition, my approach is compatible with most educational methods and philosophies which means it will be easy for you to find a way in which we can work on things together. In addition, my long experience in the educational field has allowed me to develop a unique approach that is both productive and enjoyable.
I have tutored in course hero for quite some time and was among the top tutors awarded having high helpful rates and reviews. In addition, I have also been lucky enough to be nominated a finalist for the 2nd annual course hero award and the best tutor of the month in may 2022.
I will make sure that any student of yours will have an amazing time at learning with me, because I really care about helping people achieve their goals so if you don't have any worries or concerns whatsoever you should place your trust on me and let me help you get every single thing that you're looking for and more.
In my experience, I have observed that students tend to reach their potential in academics very easily when they are tutored by someone who is extremely dedicated to their academic career not just as a businessman but as a human being in general.
I have successfully tutored many students from different grades and from all sorts of backgrounds, so I'm confident I can help anyone find the solution to their problems and achieve
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The reaction of chloroethane with water in the gas phase to produce ethanol and hydrogen chloride has Ho = +26.6 kJ mol-1 and So = +4.81 J K-1 mol-1 at 25oC. (a) Which of these terms, if either,...
-
The decomposition of acetaldehyde was studied in the gas phase at 791 K. The results of the measurements are shown as follows: Initial concentration (in mol/L) 9.72x10 -3 4.56x10 -3 Half-life (in s)...
-
Elemental nitrogen exists as N2, whereas in the gas phase the elements phosphorus, arsenic, and antimony consist of P4, As4, and Sb4 molecules, respectively. Give a possible reason for this...
-
j) Assume that one of these portfolio's is the Market Portfolio and all portfolios, except Portfolio G, are fairly priced according to the CAPM. Derive the Treynor Measure for these fairly priced...
-
Fiero Products, LTD, of Bologna, Italy, makes a variety of footwear, including indoor slippers, childrens shoes, and flip-flops. To keep up with increasing demand, it is considering three expansion...
-
Would the 50 employees be considered a population or a sample? PlayTime Toys Inc. employs 50 people in the Assembly Department. Of the employees, 40 belong to a union, and 10 do not. Five employees...
-
This appeal involves the validity of a will executed in contravention of an earlier contract to make mutual wills. A husband and wife signed a contract to make mutual wills and then executed those...
-
Near the end of 2011, the management of Simid Sports Co., a merchandising company, prepared the following estimated balance sheet for December 31, 2011. To prepare a master budget for January,...
-
Explain entity integrity and referential integrity rules in relational model. Show how these are realized in SQL.
-
A 1 The standard cost card for a single unit of Robinson, Inc.'s products is shown below. 2 3 4 Direct materials: 5 Direct labor: 6 Variable overhead (based on labor hours): 7 8 Budgeted production...
-
The light-induced electron transfer reactions in photosynthesis occur because chlorophyll molecules (whether in monomeric or dimeric forms) are better reducing agents in their electronic excited...
-
The photochemical chlorination of chloroform in the gas has been found to follow the rate law d[CCl 4 ]/dt = k[Cl 2 ] 1/2 I a 1/2 . Devise a mechanism that leads to this rate law when the chlorine...
-
Indicate the metric unit of measurement you would use to express the area of the following. A kitchen table top.
-
Suppose that Mahesh is a typical Buffalo Bills fan, and his demand curve for Bills football games is: P = 120 10G where G is the number of games the fan attends. a. If the Bills want to sell him a...
-
Suppose that the WNBAs Los Angeles Sparks raise ticket prices from $50 to $60 per seat and experience a 5 percent decline in tickets sold. What is the elasticity of demand for tickets?
-
In the late 1930s and early 1940s, Joe Louis handily defeated a series of opponents who came to be known as the Bum of the Month Club. Use what you know about rank-order tournaments to explain how...
-
Suppose the NBPA had staged a strike at the depths of the Great Recession in 2009. What would economic conditions have done to the contract zones of the NBA and the NBPA?
-
Why was the NFL able to exclude black players in the 1930s? Why did this color line collapse so quickly in the early 1950s?
-
Consider an experiment with four groups, with eight values in each. For the ANOVA summary table below, fill in all the missing results: Mean Degrees of Sum of Square Source Freedom Squares (Variance)...
-
The cash records of Holly Company show the following four situations. 1. The June 30 bank reconciliation indicated that deposits in transit total $720. During July, the general ledger account Cash...
-
On the assumption that the tension required to keep a sample at a constant length is proportional to the temperature (t = aT, the analogue of p T), show that the tension can be ascribed to the...
-
Radius of gyration is defined in eqn 19.32. Show that an equivalent definition is that Rg is the average root mean square distance of the atoms or groups (all assumed to be of the same mass), that...
-
The pure rotational microwave spectrum of HCI has absorption lines at the following wave numbers (in cm-1): 21.19, 42.37, 63.56, 84.75, 105.93, 127.12148.31169.49,190.68,211.87,233.06,254.24, 275.43,...
-
If you were given $10,000 to start a business or to develop a new product or service, what type of business would you start, or what type of product or service would you offer? Use at least one...
-
When will an investment of $10,000 growing by 5% per year be larger in value that an investment of $12,000 that is growing by only 3% per year?
-
1. Prices in a market are determined by the conditions of demand and supply and these conditions can change fo a number of different reasons at any time. (1) Describe what is meant by demand. (2)...

Study smarter with the SolutionInn App


