The molar heat capacity C P,m of SO 2 (g) is described by the following equation over
Question:
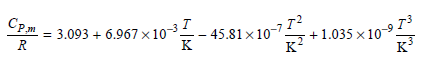
In this equation, T is the absolute temperature in kelvin. The ratios T€/K€ ensure that CP,m has the correct dimension. Assuming ideal gas behavior, calculate q, w, ΔU, and ΔH if 1.50 moles of SO2(g) is heated from 22.5°C to 1140.°C at a constant pressure of 1 bar. Explain the sign of w.
Transcribed Image Text:
Сри 3.093 + 6.967 x103÷- 45.81x10-7 -2 +1.035 ×10 K- 9. K K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
858 10 4 J U H PV H nRT 858 10 4 J 150 mol 8314 J mol 1 K 1 141315 K 29565 K 718 10 4 J W U ...View the full answer

Answered By

Muhammad Ahtsham Shabbir
I am a professional freelance writer with more than 7 years’ experience in academic writing. I have a Bachelor`s Degree in Commerce and Master's Degree in Computer Science. I can provide my services in various subjects.
I have professional excellent skills in Microsoft ® Office packages such as Microsoft ® Word, Microsoft ® Excel, and Microsoft ® PowerPoint. Moreover, I have excellent research skills and outstanding analytical and critical thinking skills; a combination that I apply in every paper I handle.
I am conversant with the various citation styles, among them; APA, MLA, Chicago, Havard, and AMA. I also strive to deliver the best to my clients and in a timely manner.My work is always 100% original. I honestly understand the concern of plagiarism and its consequences. As such, I ensure that I check the assignment for any plagiarism before submission.
4.80+
392+ Reviews
587+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
In a certain polytropic process the volume of argon was in- creased a- 4.0 times. Simultaneously, the pressure decreased = 8.0 times. Find the molar heat capacity of argon in this process, assuming...
-
The molar heat capacity of a liquid is normally greater than its vapor-phase heat capacity at modest pressure and the same temperature. Why?
-
The heat capacity at constant volume of hydrogen sulfide at low pressures is C v [kJ/ (mol??C)] = 0.0252 + 1.547 x 10 ?5? T ? 3.012 X 10 ?9 T 2? where T is in ?C. A quantity of H 2 S is kept in a...
-
Graph the function y = (x + |x|). At what values of x does the derivative exist and what is the value of the derivative when it does exist?
-
When is the "Income Summary" account used? Assume there is a net loss for the period. Make all journal entries affecting Income Summary. You do not need to include numbers or specific account titles...
-
An air-track glider undergoes a perfectly inelastic collision with an identical glider that is initially at rest. What fraction of the first glider's initial kinetic energy is transformed into...
-
The probabilities that a TV station will receive \(0,1,2,3, \ldots, 8\) or at least 9 complaints after showing a controversial program are, respectively,...
-
Rank the following securities from lowest (1) to highest (8) in terms of their riskiness for an investor. All securities (except the Treasury bond) are for a given firm. If you think two or more...
-
What are the FASB codifications regarding auction rate securities, collateralized debt obligations, fixed-for-float OTC gas swap, and auction rate securities?
-
Write a function to add two floating point numbers. Determine the integer floor of the sum. The floor is the truncated float value, i.e. anything after the decimal point is dropped. For instance,...
-
Predict the geometry of each atom except hydrogen in the compounds below: a. b. c. d. - - I0 I0 :0I
-
Use the relation (U/V ) T = T(P/T) V P and the cyclic rule to obtain an expression for the internal pressure, (0U/0V )T , in terms of P, , T, and .
-
Refer to the data in problem 14-B3. Prepare summary journal entries for the use of direct materials and conversion costs. Also, prepare a journal entry for the transfer of goods completed, assuming...
-
After you have studied Economics in the News (pp. 236237), answer the following questions. a. If after the pandemic the prices of streaming services doubled with an unchanged price of a movie ticket:...
-
Starbucks has more than 10,000 employees in 6,700 stores, which serve some 50 million customers in 51 countries each week. Geoffrey Mwa Ngulumbi runs a family-owned fair-trade farm in Tanzania and...
-
Cindy loses her math tutoring job and the amount she has to spend on golf and tennis falls from $70 to $35 a month. With the price of an hour of golf at $10 and of tennis at $5, calculate the change...
-
Lees accountant recorded the depreciation on his cottage during 2021 as $7,000. According to the accountant, what profit did Lee make? Lee is a computer programmer who earned $35,000 in 2020. But on...
-
Calculate Lees opportunity cost of production and his economic profit. Lee is a computer programmer who earned $35,000 in 2020. But on January 1, 2021, Lee opened a body board manufacturing business....
-
If f() = cos and f(a) = 1/4, find the exact value of: (a) f(-a) (b) f(a) + f(a + 2) + f(a 2) Use the periodic and evenodd properties.
-
For the following exercises, find the inverse of the function and graph both the function and its inverse. f(x) = 4 x 2 , x 0
-
The equilibrium A ( B is first -order in both directions. Derive an expression for the concentration of A as a function of time when the initial molar concentrations of A and Bare [A]0 and [B]0. What...
-
Derive the integrated form of a third-order rate law v = k[A f [B] in which the stoichiometry is 2 A + B ( P and the reactants are initially present in (a) Their stoichiometric proportions, (b) With...
-
Show that the ratio t1/2/t3/4 where 1112 is the half-life and 13/4is the time for the concentration of A to decrease to t of its initial value (implying that t3/4 < t1/2) can be written as a function...
-
Based on the introductory in Public Finance, what do you think must be the main role of the government in our individual lives? Any references will do, as long as the answer is related to question...
-
Apercu Industries borrowed $92,350 for 200 days at 15% simple interest. Find the total interest they will pay (a) using bankers' rule and (b) using the exact method.
-
On January 4th, Mark went to the hospital to have an appendectomy. His deductible is $2,500 and since it is the beginning of the year, he has not contributed anything to the deductible yet. The total...

Study smarter with the SolutionInn App


