The rate constant of the reaction I (aq) + H 2 O 2 (aq) H
Question:
The rate constant of the reaction I−(aq) + H2O2(aq) → H2O(l) + IO−(aq) varies slowly with ionic strength, even though the Debye–Hückel limiting law predicts no effect. Use the following data from 25°C to find the dependence of log kr on the ionic strength:
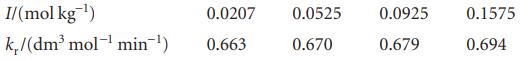
Evaluate the limiting value of kr at zero ionic strength. What does the result suggest for the dependence of log γ on ionic strength for a neutral molecule in an electrolyte solution?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





