The vapor pressure of a liquid can be written in the empirical form known as the Antoine
Question:
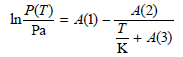
Starting with this equation, derive an equation giving ΔH Vaporization m as a function of temperature.
Transcribed Image Text:
A(2) A(1) – - + A(3) P(T) In- т Pa к
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
For a liquidgas equilibrium ...View the full answer

Answered By

Deborah Joseph
My experience has a tutor has helped me with learning and relearning. You learn everyday actually and there are changes that are made to the curriculum every time so being a tutor has helped in keeping me updated about the present curriculum and all.
I have also been able to help over 100 students achieve better grades particularly in the categories of Math and Biology both in their internal examinations and external examinations.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The conversion of the kinetic energy of wind to electricity may be an attractive alternative to the use of fossil fuels. Typically, wind causes the rotor of a turbine to turn, and a generator...
-
The Arnold Diffusion Cell shown in Figure 26.5 is a simple device used to measure gas-phase diffusion coefficients for volatile substrates in air. In the present experiment, liquid acetone is loaded...
-
In this problem, you calculate the error in assuming that ÎH o R is independent of T for the reaction 2CuO(s) 2Cu(s) + O 2 (g). The following data are given at 25°C:
-
Problem 9- 3A Estimating and reporting bad debts P2 At December 31, 2013, Hawke Company reports the following results for its calendar year. Cash sales . . . . . . . . . . $ 1,905,000 Credit sales ....
-
1. Using ERG theory, explain the reasons for the situation described in the case. Existence needs (E): Relatedness needs (R): Growth needs (G): 2. Using expectancy theory, explain the reasons for the...
-
The salaries (in millions of dollars) for 31 NFL teams for a specific season are given in this frequency distribution. Class limits Frequency 39.942.8 ........2 42.945.8 ........2 45.948.8 ........5...
-
What is a pump? What are its functions?
-
Superb Drive-Ins borrowed money by issuing $6,000,000 of 4% bonds payable at 97.5. Requirements 1. How much cash did Superb receive when it issued the bonds payable? 2. How much must Superb pay back...
-
Love Company's accounting records show an after-closing balance of $20,900 in its Retained Earnings account on December 31, Year 2. During the Year 2 accounting cycle, Love earned $18,100 of revenue,...
-
Explain why each of these is critical for a successful supply chain operation: a. Integrated technology b. Information sharing c. Trust among trading partners d. Real-time information availability e....
-
Calculate the vapor pressure for a mist of spherical water droplets of radius a. 1.95 10 8 m b. 2.25 10 6 m at 298 K. The vapor pressure of water at this temperature is 25.2 Torr.
-
Use the following vapor pressures of propane given here to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P (Torr) T (K) 0.01114 100. 120 2.317...
-
In Exercises use differentials and the graph of g' to approximate (a) g(2. 93) (b) g(3. 1) given that g(3) = 8 3 2 1 y g' 2 (3, -1/-) 4 5 X
-
Jimmy deposits $4,000 now, $2,500 three years from now, and $5,000 six years from now. Interest is 5 percent for the first 3 years and 7 percent for the last 3 years. a. How much money will be in the...
-
Based on Exhibit 1, what capital market effect is Country Z most likely to experience in the short-term? A. Cyclical assets attract investors. B. Monetary policy becomes restrictive. C. The yield...
-
State the four DCF rules.
-
You have $2,000 that you want to invest at the beginning of each of 5 years. The following alternatives are available to you: An investment that pays 7 percent for year 1, 6 percent for year 2, 5...
-
If you invest \($2\),000 today, \($3\),000 in 2 years, \($4\),000 in 5 years, and \($1\),000 in 7 years, how much will be in the bank 15 years from today if interest is 6 percent compounded annually?
-
Explain when a professionals liability will be based on contract and when it will be based on tort. How is the standard imposed with respect to tort determined?
-
Using thermodynamic data from Appendix 4, calculate G at 258C for the process: 2SO 2 (g) + O 2 (g) 88n 2SO 3 (g) where all gases are at 1.00 atm pressure. Also calculate DG8 at 258C for this same...
-
Find theLaplace transform of the function f(t) = t n e at , where n is an integer.
-
Find the Laplace transform of sin 2 (at).
-
Derive the version of Eq. (11.49) for n = 2.
-
Siran plans to contribute $850 at the start of every half year to an investment that ears 7% compounded monthly. If Siran starts contributing on his 37th birthday, how much will he accumulate by his...
-
Milwaukee Insurance Company (MIC) has entered into a four-year plain vanilla swap with a counterparty. The notional principal is $80 million. MIC will pay payments based on a floating rate of the...
-
Newton Bay is a sparsely populated area. The government plans to build an international airport there and announces this plan publicly. When hotel chains learn about the airport, they become...

Study smarter with the SolutionInn App


