Question: This problem gives a simple example of a quantitative structure activity relation (QSAR). The binding of nonpolar groups of amino acid to hydrophobic sites in
This problem gives a simple example of a quantitative structure– activity relation (QSAR). The binding of nonpolar groups of amino acid to hydrophobic sites in the interior of proteins is governed largely by hydrophobic interactions.
(a) Consider a family of hydrocarbons ReH. The hydrophobicity constants, π, for RaCH3, CH2CH3, (CH2)2CH3, (CH2)3CH3, and (CH2)4CH3 are, respectively, 0.5, 1.0, 1.5, 2.0, and 2.5. Use these data to predict the π value for (CH2)6CH3.
(b) The equilibrium constants KI for the dissociation of inhibitors (9) from the enzyme chymotrypsin were measured for different substituents R:

Plot log KI against π. Does the plot suggest a linear relationship? If so, what are the slope and intercept to the log KI axis of the line that best fits the data?
(c) Predict the value of KI for the case R=H.
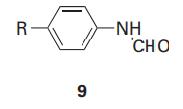
R R log K CH,CO -0.20 -1.73 CN -0.025 -1.90 NO 0.33 -2.43 CH3 0.5 -2.55 0.9 -3.40
Step by Step Solution
3.28 Rating (154 Votes )
There are 3 Steps involved in it
a The hydrophobicity constants for a family of hydrocarbons ReH can be related to the number of CH... View full answer

Get step-by-step solutions from verified subject matter experts


