Two empirical equations of state of a real gas are as follows: Evaluate (S/V) T for each
Question:
Two empirical equations of state of a real gas are as follows:
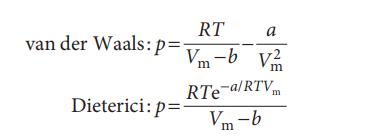
Evaluate (∂S/∂V)T for each gas. For an isothermal expansion, for which kind of gas (also consider a perfect gas) will ΔS be greatest? Explain your conclusion.
Transcribed Image Text:
RT a van der Waals: p=V-b V m m RTe-a/RTVm Dieterici: p= V-b
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
For the van der Waals gas we have SV pVT pTV SV RTVmbVm2bVm aRTVm2bVm SV ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
Consider the isothermal expansion of 1.00 mole of ideal gas at 27oC. The volume increases from 30.0 L to 40.0 L. Calculate q, w, E, H, S, and G for two situations: a. a free expansion b. a reversible...
-
A quantity of an ideal gas undergoes an isothermal expansion at 20 oC and does 3.0 x 103 J of work on its surroundings in the process. (a) Will the entropy of the gas (1) increase, (2) remain the...
-
Explain how the perfect gas equation of state arises by combination of Boyle's law, Charles's law, and Avogadro's principle.
-
What do you think people would say about Corrie from the few quotes we have from her book? What was her personality like? Do you think she handled her incarceration differently than Elie Wiesel?...
-
Summa Manufacturing Company issued $ 900,000 par value, 5%, five- year bonds dated January 1, 2016. The bonds pay interest semiannually each June 30 and December 31. Summa issued the bonds on April...
-
The 8-lb body of Prob. 8/25 is released from rest a distance x 0 to the right of the equilibrium position. Determine the displacement x as a function of time t, where t = 0 is the time of release. c...
-
In 1940, the family of Thomas Back entered into an oil-and-gas lease with the Inland Gas Corporation. The lease held that Inland would pay to Backs family 12 cents per thousand cubic feet of gas...
-
Catlet Co. uses a periodic inventory system. Its records show the following for the month of May, in which 65 units were sold. InstructionsCompute the ending inventory at May 31 and cost of goods...
-
Use the below table to answer the following questions. Selling Price $43.00 = Sales Volume Variable 2,200 3,200 Fixed Cost Cost 4,200 Profitability 5,200 6,200 $47,200 15 $14,400 $42,400 $70,400...
-
Information about Zhu Boards is presented in E6-4. Additional data regarding Zhu's sales of Xpert snowboards are provided below. Assume that Zhu uses a perpetual inventory system. Instructions (a)...
-
Which of F 2 (g) and I 2 (g) is likely to have the higher standard molar entropy at 298K?
-
Discuss the relationships between the various formulations of the Second Law of thermodynamics.
-
Marty Co. closes its books monthly. On June 30, selected ledger account balances are: Notes Receivable... $57,000 Interest Receivable 420 Notes Receivable include the following. During July, the...
-
Question 10 (5 points) Your code is using a symbol table of type BinarySearchST . What methods must be defined in classes Account and/or Transactions in order for the symbol table to work correctly?...
-
Combined Furniture Warehouse received an invoice of $8000 dated November 3rd with terms 2/10, 1/20, n/30. Combined Furniture made a payment of $2,940 on November 12th. How much did Combined Furniture...
-
Of the $120 billion spent by the Federal Housing Administration and the Veterans Administration in paying for hew homes from 1934 to 1962, what amount went to white families? Why and how?
-
Well Co. has a $20,000 receivable from Eight Co. and a $40,000 receivable from Cotten Corporation. Well also has a $30,000 payable to Sloane Co. Well owns 80% of Eight, 45% of Cotten, and 55% of...
-
When creating an executive compensation plan, which factor should the HR leader consider as critical? Why and how?
-
A control system with a controller is shown. Select KP and Kf so that the overshoot to a step input is equal to 5% and the velocity constant Kv is equal to 5. Verify the results of your design.
-
A handrail, which weighs 120 N and is 1.8 m long. was mounted to a wall adjacent to a small set of steps (Figure P4.26). The support at A has broken, and the rail has fallen about the loose bolt at 8...
-
Discuss the steps involved in the construction of sp 3 , sp 2 , and sp hybrid orbitals.
-
Give the ground-state electron configurations and bond orders of (a) Li 2 , (b) Be 2 , and (c) C 2 .
-
The overlap integral between two H1 s orbitals on nuclei separated by a distance R is S = {1 + (R/a 0 ) + 1/3 (R/a 0 ) 2 }e R/a0 . Plot this function for 0 R < .
-
Joey, whose mass is m = 36 kg, stands at rest at the outer edge of a frictionless merry-go-round with the mass M = 300 kg and the radius R = 2.0 m, as in. The merry-go-round is also at rest. Joey...
-
The transaction gain or loss in the consolidated financial statements is reported as a foreign currency transition adjustment. In this case, since the transaction gain or loss is negative then it...
-
a) Assume this tunnel is dug from New York to San Francisco, a distance of 5000 km measured along the surface. A car rolling on steel rails is released from rest at New York, and rolls through the...

Study smarter with the SolutionInn App


