Question: Why do we neglect the bond length in He 2 when discussing the trends shown in Figure 23.20? Figure 23.20 O, , Liz Be, B2
Figure 23.20
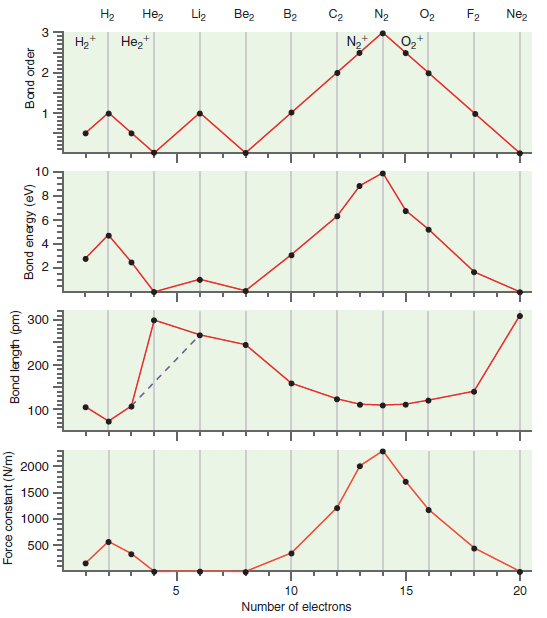
O, , Liz Be, B2 C2 N, F2 Nez N, * 300 200 100 10 15 20 Number of electrons Force constant (N/m) Bond length (pm) Bond energy (eV) Bond order 2. 2.
Step by Step Solution
3.38 Rating (176 Votes )
There are 3 Steps involved in it
Because the bond in He is not a chemical bond ... View full answer

Get step-by-step solutions from verified subject matter experts


