You have been assigned the task of measuring the equilibrium constant for the reaction N 2 O
Question:
You have been assigned the task of measuring the equilibrium constant for the reaction N2O4 ⇌2NO2 as a function of temperature. To do so, you evacuate a rigid 2-liter vessel equipped with a pressure gauge, fill the vessel with a mixture of NO2 and N2O4, and then heat it to T0 473 K, a temperature at which you know the gas is essentially pure NO2. The gauge pressure at this point is noted to be 1.00 atm. You then decrease the temperature in stages, recording the equilibrium gauge pressure at each temperature. The data are as follows:

(a) How many gram-moles of NO2 are in the vessel at 473 K?
(b) The reaction equilibrium constant is
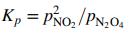
where pNO2 and pN2O4 are the equilibrium partial pressures of NO2 and N2O4. Derive an equation or a series of equations for calculating Kp(atm) from specified values of T and Pgauge. (Suggestion: Begin by defining n1 and n2 as the moles of NO2 and N2O4 present at equilibrium.) Then calculate Kp for T 350 K, 335 K, 315 K, and 300 K. (Suggestion: Use a spreadsheet.)
(c) The equilibrium constant should vary with temperature according to the relation
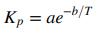
Use the results of Part (b) to determine the values of a and b by curve-fitting.
Methanol is synthesized from carbon monoxide and hydrogen in the reaction
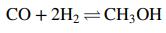
A process flowchart is shown below.
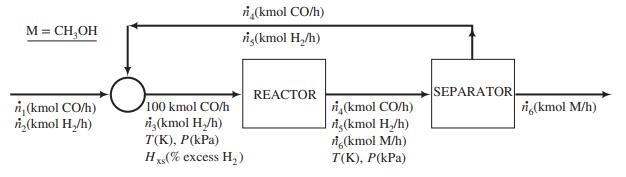
The fresh feed to the system, which contains only CO and H2, is blended with a recycle stream containing the same species. The combined stream is heated and compressed to a temperature T(K) and a pressure P(kPa) and fed to the reactor. The percentage excess hydrogen in this stream is Hxs. The reactor effluent—also at T and P—goes to a separation unit where essentially all of the methanol produced in the reactor is condensed and removed as product. The unreacted CO and H2 constitute the recycle stream blended with the fresh feed.
Provided that the reaction temperature (and hence the rate of reaction) is high enough and the idealgas equation of state is a reasonable approximation at the reactor outlet conditions (a questionable assumption), the ratio
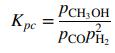
approaches the equilibrium value, which is given by the expression
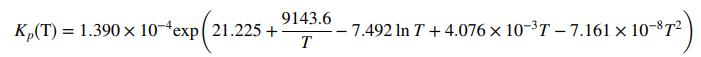
In these equations, pi is the partial pressure of speciesi in kilopascals (i = CH3OH, CO, H2) and T is in Kelvin.
(a) Suppose P = 5000 kPa, T = 500 K, and the percentage excess of hydrogen in the feed to the reactor (Hxs) = 5.0%. Calculate ṅ4; ṅ5, and ṅ6, the component flow rates (kmol/h) in the reactor effluent. [Suggestion: Use the known value of Hxs , atomic balances around the reactor, and the equilibrium relationship, Kpc = Kp(T), to write four equations in the four variables ṅ3 to ṅ6; use algebra to eliminate all but ṅ6; and use Goal Seek or Solver in Excel to solve the remaining nonlinear equation for ṅ6.] Then calculate component fresh feed rates (ṅ1 and ṅ2) and the flow rate (SCMH) of the recycle stream.
(b) Prepare a spreadsheet to perform the calculations of Part (a) for the same basis of calculation (100 kmol CO/h fed to the reactor) and different specified values of P(kPa), T(K), and Hxs(%). The spreadsheet should have the following columns:
A. P(kPa)
B. T(K)
C. Hxs(%)
D. Kp(T) 108 . (The given function of T multiplied by 108. When T = 500 K, the value in this column should be 91.113.)
E. KpP2
F. ṅ3. The rate (kmol/h) at which H2 enters the reactor.
G. ṅ4. The rate (kmol/h) at which CO leaves the reactor.
H. ṅ5. The rate (kmol/h) at which H2 leaves the reactor.
I. ṅ6. The rate (kmol/h) at which methanol leaves the reactor.
J. ṅtot. The total molar flow rate (kmol/h) of the reactor effluent.
K. Kpc 108 .
The ratio yM= (yCOy2H2) multiplied by 108. When the correct solution has been attained, this value should equal the one in Column E.
L. KpP2 - KpcP2 . Column E–Column K, which equals zero for the correct solution.
M. ṅ1. The molar flow rate (kmol/h) of CO in the fresh feed.
N. ṅ2. The molar flow rate (kmol/h) of H2 in the fresh feed.
O. V̇rec(SCMH). The flow rate of the recycle stream in m3 (STP)/h.
When the correct formulas have been entered, the value in Column I should be varied until the value in Column L equals 0.
Run the program for the following nine conditions (three of which are the same):
• T = 500 K, Hxs = 5%, and P = 1000 kPa, 5000 kPa, and 10,000 kPa.
• P = 5000 kPa, Hxs = 5%, and T = 400 K, 500 K, and 600 K.
• T = 500 K, P = 5000 kPa, and Hxs = 0%, 5%, and 10%.
Summarize the effects of reactor pressure, reactor temperature, and excess hydrogen on the yield of methanol (kmol M produced per 100 kmol CO fed to the reactor).
(c) You should find that the methanol yield increases with increasing pressure and decreasing temperature. What cost is associated with increasing the pressure?
(d) Why might the yield be much lower than the calculated value if the temperature is too low?
(e) If you actually ran the reaction at the given conditions and analyzed the reactor effluent, why might the spreadsheet values in Columns F–M be significantly different from the measured values of these quantities? (Give several reasons, including assumptions made in obtaining the spreadsheet values.)
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





