Steam reforming is an important technology for converting refined natural gas, which we take here to be
Question:
Steam reforming is an important technology for converting refined natural gas, which we take here to be methane, into a synthesis gas that can be used to produce a variety of other chemical compounds. For example, consider a reformer to which natural gas and steam are fed in a ratio of 3.5 moles of steam per mole of methane. The reformer operates at 18 atm, and the reaction products leave the reformer in chemical equilibrium at 875°C. The steam reforming reaction is

and the water–gas shift reaction also occurs in the reformer.

The equilibrium constants for these two reactions are given by the expressions
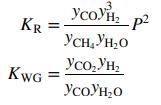
At 875°C, KR = 872:9 atm2 and KWG = 0:2482. The process is to produce 100.0 kmol/h of hydrogen. Calculate the feed rates (kmol/h) of methane and steam and the volumetric flow rate (m3/min) of gas leaving the reformer.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





