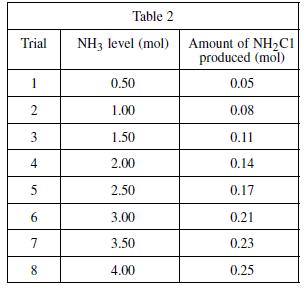
Based on the results of Experiment 3, as the NH 3 level increased from 0.50 to 4.00
Question:
Based on the results of Experiment 3, as the NH3 level increased from 0.50 to 4.00 mol, the greatest increase in the amount of NH2Cl produced occurred:
F. Between the trials of 0.50 and 1.00 mol of NH3.
G. Between the trials of 1.50 and 2.00 mol of NH3.
H. Between the trials of 2.50 and 3.00 mol of NH3.
J. Between the trials of 3.50 and 4.00 mol of NH3.
Experiment 3
In yet another reaction, bleach and ammonia combined under certain conditions produce a compound known as chloramine. Chloramine (NH2Cl) is a toxic substance commonly used in low concentrations as a disinfectant in municipal water systems as an alternative to chlorination. To determine the mixture of bleach and ammonia at which NH2Cl is produced, a varying amount of ammonia was added to eight different bleach–water solutions and the resulting chlorine gas from each mixture was collected and measured. A solution of 1.0 mole (mol) of NaOCl in 1 kg of water was used in each trial. A certain quantity of NH3 was added to each solution; the quantity of ammonia added was gradually increased for each trial. The amount of chloramine produced in each trial was recorded in Table 2.
Step by Step Answer:






