(a) The cell in Figure 17-4 is: Cu(s) |1.0 M CuSO 4 (aq) A KCl (aq, 3...
Question:
(a) The cell in Figure 17-4 is: Cu(s) |1.0 M CuSO4(aq) A KCl (aq, 3 M) | AgCl(s) | Ag(s) Write half-reactions for this cell. Neglecting activity coefficients and the junction potential between CuSO4(aq) and KCl (aq), predict the equilibrium (zero-current) voltage expected when the Luggin capillary contacts the Cu electrode. For this purpose, suppose that the reference electrode potential is 0.197 V vs. S.H.E. Why is the observed equilibrium potential 1109 mV, not the value you calculated?
(b) How would the overpotentials change if .1.000 V were imposed by the potentiostat?
Figure 17-4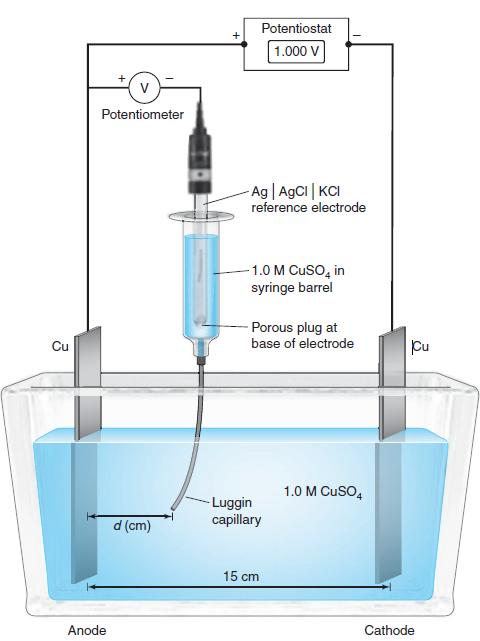
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





