Question: Evaluate E o ' for the half-reaction (CN)2(g) + 2H* + 2e = 2HCN(aq) Cyanogen Hydrogen cyanide
Evaluate Eo' for the half-reaction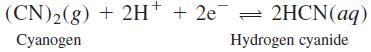
(CN)2(g) + 2H* + 2e = 2HCN(aq) Cyanogen Hydrogen cyanide
Step by Step Solution
3.31 Rating (172 Votes )
There are 3 Steps involved in it
C 2 N 2 g 2 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
2112_61d6ac346dee0_875472.pdf
180 KBs PDF File
2112_61d6ac346dee0_875472.docx
120 KBs Word File


