Standard addition with confidence interval. Ammonia in seawater was measured with an ammonia-selective electrode. A 100.0 mL
Question:
Standard addition with confidence interval. Ammonia in seawater was measured with an ammonia-selective electrode. A 100.0 mL aliquot of seawater was treated with 1.00 mL of 10 M NaOH to convert NH+4 to NH3. Therefore, V0 = 101.0 mL. A reading was then taken with the electrode. Then 10.00-mL aliquots of standard NH+4 Cl2 were added and results are shown in the table.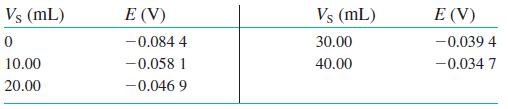
The standard contains 100.0 ppm (mg/L) of nitrogen in the form of NH+4 Cl2. A separate experiment determined that the electrode slope bRT (ln 10)/F is 0.056 6 V.
(a) Prepare a standard addition graph. Find the concentration and 95% confidence interval for ammonia nitrogen (ppm) in the 100.0 mL of seawater.
(b) Standard addition is best if the additions increase analyte to 1.5 to 3 times its original concentration. Does this experiment fall in that range? A criticism of this experiment is that too much added standard creates error because the standards contribute too much to the computed result and the reading from the initial solution is not weighted heavily enough.
Step by Step Answer:






