The following cell was constructed to fi nd the difference in Ksp between two naturally occurring forms
Question:
The following cell was constructed to fi nd the difference in Ksp between two naturally occurring forms of CaCO3(s), called calcite and aragonite.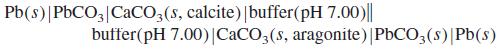
Each compartment of the cell contains a mixture of solid PbCO3 (Ksp = 7.4 × 10-14) and either calcite or aragonite, both of which have Ksp < 5 × 10-9. Each solution was buffered to pH 7.00 with an inert buffer, and the cell was completely isolated from atmospheric CO2. The measured cell voltage was 21.8 mV. Find the ratio of solubility products, Ksp (for calcite)/Ksp (for aragonite)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





